Ring Opening of Diketene in Superacidic Media
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The reactions of diketene were investigated in the HF/MF5 and DF/MF5 (M = As or Sb) binary superacidic systems. Depending on the stoichiometric ratio of the Lewis acids and diketene, monoprotonated acetoacetyl fluoride and diprotonated acetoacetyl fluoride were obtained. The salts were characterized by low-temperature vibrational spectroscopy, nuclear magnetic resonance spectroscopy, and single-crystal X-ray diffraction. [CH3C(OH)CH2COF][SbF6]·HF crystallizes in monoclinic space group P21/n with four formula units per unit cell, and [CH3C(OH)CH2C(OH)F][SbF6]2·HF in triclinic space group P1 with two formula units per unit cell. Ring opening is discussed together with quantum chemical pKa calculations. Monoprotonated acetoacetyl fluoride is characterized by a six-membered ring-like structure containing an intramolecular hydrogen bond, whereas the diprotonated species constitutes a 1,3-gitonic superelectrophile.
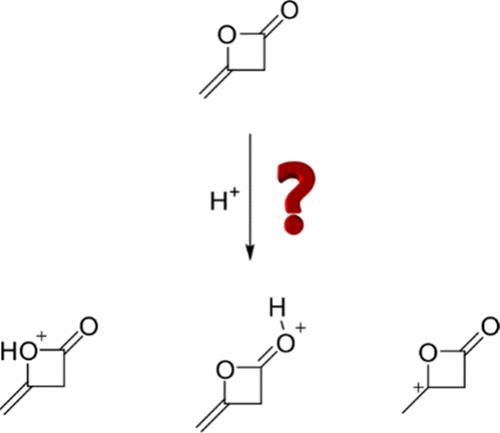
研究了二酮烯在 HF/MF5 和 DF/MF5(M = As 或 Sb)二元超酸性体系中的反应。根据路易斯酸和二酮烯的化学计量比,得到了单质子化乙酰乙酰氟和双质子化乙酰乙酰氟。这些盐通过低温振动光谱、核磁共振光谱和单晶 X 射线衍射进行了表征。[CH3C(OH)CH2COF][SbF6]-HF 结晶于单斜空间群 P21/n,每个单胞有四个式单元;[CH3C(OH)CH2C(OH)F][SbF6]2-HF 结晶于三斜空间群 P1,每个单胞有两个式单元。本文结合量子化学 pKa 计算讨论了开环问题。单质子化乙酰乙酰氟的特点是具有六元环状结构,其中包含一个分子内氢键,而双质子化的乙酰乙酰氟则是一个 1,3-硝基超亲电体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


