Synthesis of Spin-Labeled α-/β-Galactosylceramides and Glucosylceramides as Electron Paramagnetic Probes
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
α-/β-Galactosylceramide (GalCer) and glucosylceramide (GlcCer) derivatives having a radical label at the 6-C-position suitable for electron paramagnetic resonance spectroscopic studies were synthesized by a diversity-oriented strategy that is highlighted by the efficient glycosylation of a lipid precursor and late-stage ceramide assembly to enable lipid diversification. The strategy was also utilized to synthesize natural α-/β-GalCers and GlcCers. Furthermore, the involved azido-intermediates are flexible platforms to access various other GalCer and GlcCer derivatives.
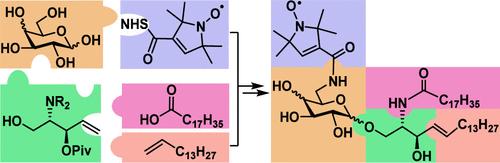
合成自旋标记的 α-/β-Galactosylceramides 和 Glucosylceramides 作为电子顺磁探针
通过一种以多样性为导向的策略合成了α-/β-半乳糖基甘油酰胺(GalCer)和葡萄糖基甘油酰胺(GlcCer)衍生物,这些衍生物在6-C位上具有适合电子顺磁共振光谱研究的自由基标记。该策略还用于合成天然的 α-/β-GalCers 和 GlcCers。此外,所涉及的叠氮中间体是获得其他各种 GalCer 和 GlcCer 衍生物的灵活平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


