A New Synthesis of Enantiopure Amine Fragment: An Important Intermediate to the Anti-HIV Drug Lenacapavir
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, we describe a new seven-step approach to prepare (S)-1-(3,6-dibromopyridin-2-yl)-2-(3,5-difluorophenyl)ethan-1-amine ((S)-4) from the inexpensive 2-(3,5-difluorophenyl)acetic acid. The key steps in the sequence include (1) the Weinreb amide-based ketone synthesis to provide an entry point to the core structure; (2) simple functional group transformations to afford the racemic amine 4-rac; and (3) dynamic kinetic resolution (DKR) to access the chiral amine (S)-4. This seven-step process delivered the enantiopure amine (S)-4 in an overall isolated yield of approximately 15%. The process was demonstrated on a decagram scale, and the process requires no chromatographic purifications. Single-crystal X-ray crystallography measurements verified the chiral amine structure and absolute configuration.
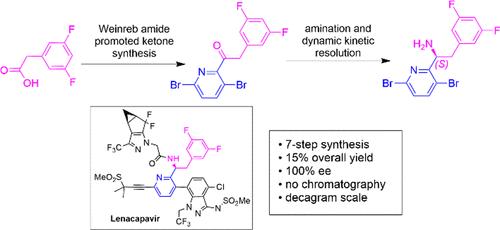
对映体纯胺片段的新合成:抗艾滋病毒药物来那卡巴韦的重要中间体
在此,我们描述了一种新的七步方法,从廉价的 2-(3,5-二氟苯基)乙酸制备 (S)-1-(3,6- 二溴吡啶-2-基)-2-(3,5-二氟苯基)乙-1-胺((S)-4)。这一系列的关键步骤包括:(1) Weinreb 酰胺基酮合成,为核心结构提供切入点;(2) 简单的官能团转化,得到外消旋胺 4-rac;(3) 动态动力学解析 (DKR),得到手性胺 (S)-4。这七步工艺可得到对映体纯胺 (S)-4,总体分离收率约为 15%。该过程已在十克规模上进行了演示,而且无需色谱纯化。单晶 X 射线晶体学测量验证了手性胺的结构和绝对构型。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


