Description of ions properties using molecular orbital energy levels: Trends in interaction with model electrodes and solvents
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
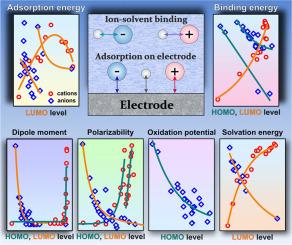
The study reveals correlations between the parameters of ions and their HOMO and LUMO orbital energy level values. In particular, it demonstrates a clear correlation for the ion adsorption parameters on model electrodes: the aluminum oxide (0001) surface, graphene and Au (111) surface. Correlations are also observed for the parameters of ion binding to water and dimethyl carbonate molecules, which are often used as solvents. In addition, the dipole moment, polarizability and solvation energy of ions are well correlated with the values of the molecular orbital energies, and for anions a dependence on the oxidation potential is observed. The experimental ion parameters reported in the literature also show correlations with the energy levels of the ion orbitals. The obtained descriptors make it possible to select ions with desired values for a specific problem. As an illustrative example, in this work we consider the problem of displacement of water molecules from the inner electric double layer by ions, which is one of the factors increasing the potential window in electrolytes of aqueous batteries. This approach can be applied in the rapidly developing field of aqueous electrolytes for battery or supercapacitor design, catalysis control through surface composition variations, as well as in studies of heavy metal ion binding to sorption materials.
用分子轨道能级描述离子性质:与模型电极和溶剂相互作用的趋势
研究揭示了离子参数与其HOMO和LUMO轨道能级值之间的相关性。特别是,它证明了模型电极上离子吸附参数的明确相关性:氧化铝(0001)表面,石墨烯和Au(111)表面。还观察到离子与水和碳酸二甲酯分子(通常用作溶剂)的结合参数的相关性。此外,离子的偶极矩、极化率和溶剂化能与分子轨道能的值有很好的相关性,而阴离子则与氧化势有关。文献中报道的实验离子参数也显示出与离子轨道能级的相关性。获得的描述符使选择具有特定问题所需值的离子成为可能。作为一个例子,在这项工作中,我们考虑了水分子被离子从内双电层置换的问题,这是增加水性电池电解质电位窗口的因素之一。这种方法可以应用于快速发展的电池或超级电容器设计的水电解质领域,通过表面组成变化的催化控制,以及重金属离子与吸附材料结合的研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


