Achieving Bright Luminescence and X-ray Scintillation in Zero-Dimensional Cesium Zinc Bromides by Cu+–Mn2+ Codoping
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Zero-dimensional (0D) metal halides have emerged as excellent luminescent materials for optical and optoelectronic applications. Especially environmentally friendly ternary zinc halides have recently drawn increasing attention. Herein, we present the codoping of Cu+ and Mn2+ ions into 0D Cs2ZnBr4 single crystals (SCs), which show bright PL emission and high stability. Adjusting the Cu+/Mn2+ ratio can make the photoluminescence quantum yield (PLQY) exceed 90%, which is much higher than that of the single-ion doped sample. The efficient PL is determined by a combination of Cu+–Mn2+ competitive interaction, Cu+–Mn2+ energy transfer, and Cu+–Mn2+ synergistic passivation. More interestingly, the codoped sample shows a better scintillation performance with a low detection limit of 52 nGyair/s and a sensitive spatial resolution of 13.2 lp/mm. We further explore the promising applications of Cu+–Mn2+-codoped Cs2ZnBr4 SCs for anticounterfeiting and X-ray imaging. These results not only help to grasp the excited-state photophysical processes of 0D codoped metal halides but also provide a new way for the design and development of environmentally friendly luminescent materials.
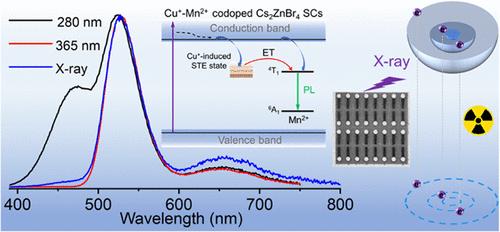
通过Cu+ -Mn2 +共掺杂实现零维铯锌溴化物的明亮发光和x射线闪烁
零维(0D)金属卤化物已成为光学和光电子应用的优秀发光材料。特别是对环境友好的三元卤化锌近年来越来越受到人们的关注。在此,我们提出了Cu+和Mn2+离子共掺杂到0D Cs2ZnBr4单晶(SCs)中,该单晶具有明亮的PL发射和高稳定性。调整Cu+/Mn2+的比例可以使光致发光量子产率(PLQY)超过90%,远高于单离子掺杂样品。有效的PL是由Cu+ -Mn2 +竞争相互作用、Cu+ -Mn2 +能量转移和Cu+ -Mn2 +协同钝化共同决定的。更有趣的是,共掺杂样品显示出更好的闪烁性能,低检测限为52 nGyair/s,敏感空间分辨率为13.2 lp/mm。我们进一步探索了Cu+ -Mn2 +-共掺杂Cs2ZnBr4 SCs在防伪和x射线成像方面的应用前景。这些结果不仅有助于掌握0D共掺杂金属卤化物的激发态光物理过程,而且为环保发光材料的设计和开发提供了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
文献相关原料
公司名称
产品信息
阿拉丁
Cesium bromide (CsBr)
阿拉丁
Zinc bromide
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


