Stereoactive Electron Lone Pairs Facilitate Fluoride Ion Diffusion in Tetragonal BaSnF4
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Solid-state ionic conductors are of primary importance for the design of tomorrow’s batteries. In lithium- or sodium-ion-based materials, the alkali cations diffuse through three-dimensional channels consisting of interconnected tetrahedral or octahedral sites with low free energy barriers between them. Fluoride ion conductors stand out in this landscape since the materials with the highest conductivities belong to the MSnF4 family (in which M2+ is a divalent cation), whose structure is layered and characterized by double-layers of Sn2+ and M2+ cations along a given direction. Importantly, these materials display stereoactive electron lone pairs (LPs) that seemingly play an important role not only in stabilizing the Sn–Sn layer but also in modulating the fluoride ion diffusive behavior. However, despite previous experimental and simulation studies, the involvement of the LPs in the fluoride ion conduction mechanism remains to be quantitatively understood. In this work, we simulate the BaSnF4 tetragonal structure using machine learning-based molecular dynamics, in which the interaction potential is trained on density functional theory data. We investigated the role of the Sn–LP–Sn layer in lowering the diffusion energy landscape. In particular, we show how the F– ions jump across this layer and occur much more frequently than in the Ba–F–Ba one, resulting in the formation of vacancies in the Ba–Sn layers. Concurrently, the LP stereochemical activity fluctuates to accommodate the F ions jumping. In addition, the presence of the LP layer enhances the flexibility of the Sn ions, which leads to an increase in two-dimensional diffusion by several orders of magnitude. These results contribute to our understanding of the interplay between LPs and ionic diffusion, helping to explain the good performance of the material in fluoride-ion batteries.
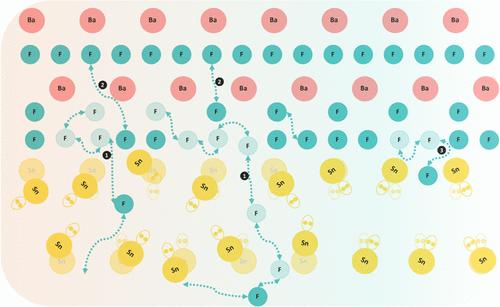
立体活性电子孤对促进四方 BaSnF4 中氟离子的扩散
固态离子导体对于未来电池的设计至关重要。在以锂离子或钠离子为基础的材料中,碱阳离子通过由相互连接的四面体或八面体位点组成的三维通道进行扩散,这些位点之间的自由能障很低。氟离子导体在这一领域脱颖而出,因为导电率最高的材料属于 MSnF4 系列(其中 M2+ 是二价阳离子),其结构呈层状,特点是沿特定方向存在 Sn2+ 和 M2+ 阳离子双层。重要的是,这些材料显示出立体活性电子孤对(LPs),似乎不仅在稳定锡-锡层方面起着重要作用,而且还能调节氟离子的扩散行为。然而,尽管之前进行了实验和模拟研究,但 LPs 在氟离子传导机制中的参与仍有待定量了解。在这项工作中,我们利用基于机器学习的分子动力学模拟了 BaSnF4 四边形结构,其中的相互作用势是根据密度泛函理论数据训练的。我们研究了 Sn-LP-Sn 层在降低扩散能谱中的作用。特别是,我们展示了 F- 离子如何跃过该层,并且比在 Ba-F-Ba 层中出现得更频繁,从而导致在 Ba-Sn 层中形成空位。与此同时,LP 的立体化学活性也在波动,以适应 F 离子的跃迁。此外,LP 层的存在增强了锡离子的柔韧性,从而使二维扩散增加了几个数量级。这些结果有助于我们理解 LP 与离子扩散之间的相互作用,有助于解释该材料在氟离子电池中的良好性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


