Suppressing organic cation reactivity in locally concentrated ionic liquid electrolytes for lithium metal batteries
IF 18.9
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
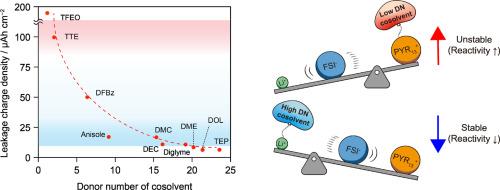
The quest for highly stable ionic liquid electrolytes is vital for longer, safer cycling of Li-metal batteries (LMBs), given their nonflammable nature and broad electrochemical window. Locally concentrated ionic liquid electrolytes (LCILEs) have emerged by incorporating anti-solvating co-solvents to address the high viscosity and poor conductivity of Li+-concentrated ionic liquids. Although solvation and interface chemistry are crucial in determining cell performance, the impacts of organic cations in LCILEs remain overlooked. This work unravels the co-solvent-guided mediation of organic cation reactivity toward Li metal anodes. The donor number (DN) of co-solvents is found to significantly influence their local distribution within LCILEs, modulating Coulombic interactions between Li+–anion complexes and organic cations. Low DN co-solvents, such as hydrofluoroethers, hardly interact with Li+–anion complexes but dissociate and destabilize organic cations, adversely promoting organic cation decomposition at Li metal anodes. Conversely, high DN co-solvents prefer to occupy the Li+ solvation sheath, promoting organic cation–anion association and mitigating the cathodic decomposition. Suppressing the reactivity of organic cations in LCILEs is essential for proper anion-derived solid-electrolyte interphase formation and stable cycling of LMBs. The controlled reactivity of organic cations in concentrated ionic liquid electrolytes incorporating high DN co-solvent enables stable cycling of LMBs under stringent conditions, achieving 95 % capacity retention over 200 cycles.

抑制局部浓缩离子液体电解质中有机阳离子的反应性
鉴于锂金属电池(LMB)的不可燃性和宽广的电化学窗口,寻求高度稳定的离子液体电解质对于锂金属电池更长、更安全的循环至关重要。局部浓缩离子液体电解质(LCILE)是通过加入抗溶解助溶剂来解决 Li+ 浓缩离子液体粘度高、导电性差的问题。虽然溶解和界面化学在决定电池性能方面至关重要,但 LCILE 中有机阳离子的影响仍被忽视。这项研究揭示了共溶剂引导的有机阳离子对锂金属阳极反应性的中介作用。研究发现,助溶剂的供体数(DN)会显著影响它们在 LCILE 中的局部分布,从而调节 Li+-阴离子复合物与有机阳离子之间的库仑相互作用。低 DN 助溶剂(如氢氟醚)几乎不会与 Li+-阴离子复合物发生作用,但会解离有机阳离子并破坏其稳定性,从而不利地促进锂金属阳极上有机阳离子的分解。相反,高 DN 助溶剂更喜欢占据 Li+ 溶剂鞘,促进有机阳离子与阴离子的结合,减轻阴极分解。抑制 LCILE 中有机阳离子的反应性对于阴离子衍生的固体电解质相间形成和 LMB 的稳定循环至关重要。在含有高 DN 助溶剂的浓缩离子液体电解质中控制有机阳离子的反应性,可使 LMB 在严格的条件下稳定循环,在 200 次循环中实现 95% 的容量保持率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


