Structure-Based Development of Novel Spiro-Piperidine ASH1L Inhibitors
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
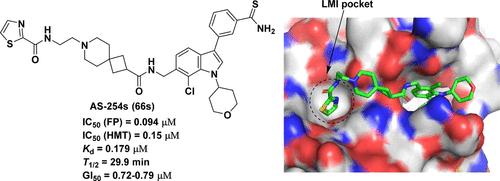
The absent, small, or homeotic-like 1 (ASH1L) protein is a histone lysine methyltransferase that plays a crucial role in various cancers, including leukemia. Despite representing an attractive therapeutic target, only one class of ASH1L inhibitors was identified to date. Herein, we report development of advanced ASH1L inhibitors targeting the catalytic SET domain, which were designed to access previously unexplored binding pocket on ASH1L. Extensive medicinal chemistry combined with structure-based design led to identification of 66s (AS-254s), a highly potent and selective ASH1L inhibitor (IC50 = 94 nM), representing substantially improved inhibitory activity over previously reported compounds targeting ASH1L. Furthermore, 66s effectively blocked cell proliferation and induced apoptosis and differentiation in leukemia cells harboring MLL1 translocations. Overall, this work provides a high-quality chemical probe targeting the catalytic SET domain of ASH1L with increased inhibitory activity and cellular efficacy to study biological functions of ASH1L and potentially to develop novel anticancer therapeutics.
新型螺旋-哌啶类ASH1L抑制剂的结构研究
缺失、小或同源样 1(ASH1L)蛋白是一种组蛋白赖氨酸甲基转移酶,在包括白血病在内的多种癌症中发挥着至关重要的作用。尽管ASH1L是一个极具吸引力的治疗靶点,但迄今为止只发现了一类ASH1L抑制剂。在此,我们报告了以催化 SET 结构域为靶点的 ASH1L 高级抑制剂的开发情况,这些抑制剂是为进入 ASH1L 上以前未探索过的结合口袋而设计的。通过广泛的药物化学研究和基于结构的设计,我们发现了 66s (AS-254s),它是一种高活性和高选择性的 ASH1L 抑制剂(IC50 = 94 nM),与之前报道的针对 ASH1L 的化合物相比,其抑制活性得到了大幅提高。此外,66s 还能有效阻止细胞增殖,并诱导携带 MLL1 易位的白血病细胞凋亡和分化。总之,这项工作提供了一种靶向 ASH1L 催化 SET 结构域的高质量化学探针,它具有更强的抑制活性和细胞功效,可用于研究 ASH1L 的生物功能,并有可能开发出新型抗癌疗法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


