Investigation and assessment of equilibrium solubility, Hansen solubility parameters and modeling of natural plant medicine artemisinin (Form Ⅰ) in three binary solvents at different temperatures
IF 5.6
1区 农林科学
Q1 AGRICULTURAL ENGINEERING
引用次数: 0
Abstract
Artemisinin has been widely used in various countries around the world for the treatment of malaria, which has saved so many lives that it continues to attract the attention of scientific researchers. However, its clinical efficacy and potential applications in other fields could be affected by its poor water-solubility. Thus, it is necessary to study the solubility and crystallization of artemisinin. At the present study, the solubility of artemisinin (Form Ⅰ) in three (tetrahydrofuran + isopropanol, 1,4-dioxane + isopropanol, 1,4-dioxane + n-propanol) binary solvents were measured from 283.15 K to 323.15 K by a gravimetric method. The Ma model, Ideal model, Jouyban-van′t Hoff model and GSM model were selected to fit the obtained solubility. Furthermore, ARD, RMSD and RD were used to evaluate the correlate reliability of the thermodynamic models. The average 100ARD and 104RMSD of the four models were 2.19 (Ma), 1.48 (GSM), 2.18 (Jouyban-van′t Hoff), 1.53 (Ideal) and 12.37 (Ma), 8.27 (GSM), 11.18 (Jouyban-van′t Hoff), 5.74 (Ideal), respectively. Additionally, DSC, TGA, PXRD and crystal morphology characterization analysis were used to analyze the crystal polymorphic change during the artemisinin dissolution process. Besides, Hansen solubility parameters and thermodynamic properties including ΔsolH°, ΔsolS°, ΔsolG°, ζH and ζTS of artemisinin (Form Ⅰ) in the selected solvents were investigated. The research could give important guiding significance and make a fundamental contribution to enrich and expand the solubility database and thermodynamic mechanism of dissolution and crystallization process of artemisinin (Form Ⅰ).
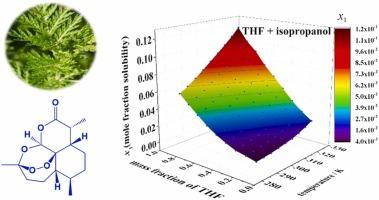
天然植物药物青蒿素(形式Ⅰ)在三种不同温度二元溶剂中的平衡溶解度、Hansen溶解度参数的研究与评价及建模
青蒿素在世界各国被广泛用于治疗疟疾,挽救了无数生命,因此一直受到科研人员的关注。然而,由于青蒿素的水溶性较差,其临床疗效和在其他领域的潜在应用可能会受到影响。因此,有必要对青蒿素的溶解性和结晶性进行研究。本研究采用重量法测定了青蒿素(Ⅰ型)在 283.15 K 至 323.15 K 三种(四氢呋喃 + 异丙醇、1,4-二氧六环 + 异丙醇、1,4-二氧六环 + 正丙醇)二元溶剂中的溶解度。选择了 Ma 模型、Ideal 模型、Jouyban-van′t Hoff 模型和 GSM 模型来拟合得到的溶解度。此外,还使用 ARD、RMSD 和 RD 来评估热力学模型的相关可靠性。四个模型的平均 100ARD、104RMSD 分别为 2.19(Ma)、1.48(GSM)、2.18(Jouyban-van′t Hoff)、1.53(Ideal)和 12.37(Ma)、8.27(GSM)、11.18(Jouyban-van′t Hoff)、5.74(Ideal)。此外,还利用 DSC、TGA、PXRD 和晶体形貌表征分析了青蒿素溶解过程中的晶体多晶型变化。此外,还考察了青蒿素(Ⅰ型)在所选溶剂中的汉森溶解度参数和热力学性质,包括ΔsolH°、ΔsolS°、ΔsolG°、ζH和ζTS。该研究对丰富和拓展青蒿素(Ⅰ型)溶解度数据库及其溶解和结晶过程的热力学机理具有重要的指导意义和基础性贡献。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial Crops and Products
农林科学-农业工程
CiteScore
9.50
自引率
8.50%
发文量
1518
审稿时长
43 days
期刊介绍:
Industrial Crops and Products is an International Journal publishing academic and industrial research on industrial (defined as non-food/non-feed) crops and products. Papers concern both crop-oriented and bio-based materials from crops-oriented research, and should be of interest to an international audience, hypothesis driven, and where comparisons are made statistics performed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


