Epitaxial Growth of Atomic-Layer Cu on Pd Nanocatalysts for Electrochemical CO2 Reduction
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
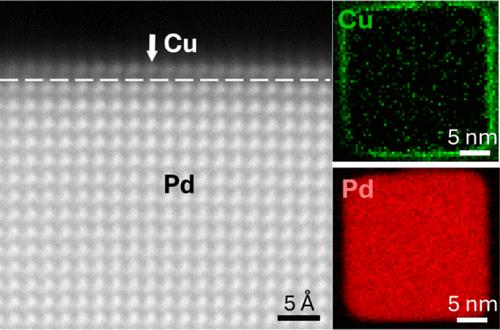
CO2 reduction reaction (CO2RR) facilitates the sustainable synthesis of fuels and chemicals. Although copper (Cu) enables CO2-to-multicarbon product (C2+) conversion, Cu-based electrocatalysts, particularly nanocatalysts, face challenges in poor selectivity and stability owing to the highly dynamic nature of Cu atoms under reaction conditions. Core–shell structures present a promising approach to address these issues by modulating the Cu overlayer–substrate interactions with atomic-level precision. Here, we report on Pd@Cu core–shell structures with atomically thin and nanometer-thick Cu overlayers on single-crystal Pd nanocubes with {100} facets promoting the CO2-to-C2+ conversion. The microstructures and surface compositions at the atomically sharp Pd/Cu interface were investigated by atomic-scale scanning transmission electron microscopy (STEM) imaging and electron energy-loss spectroscopy (EELS). Our results reveal that atomic-layer Cu epitaxially grows on Pd and adapts to the lattice of the Pd substrate. The reaction-driven migration of atomic-layer Cu is effectively suppressed on Pd due to the strong Cu–Pd interaction. While Pd only reduces CO2 to C1 products, atomic-layer Cu on Pd can initiate the C2+ production during the CO2RR. Thick Cu overlayers (∼15 nm) on Pd further enhance the C2+ faradaic efficiency while undergoing significant structural reconstruction, with only the 2–3 nm Cu layers near the Pd surface remaining stable and resistant to Cu migration after the CO2RR. We anticipate that Pd@Cu core–shell structures with intermediate Cu shell thickness hold significant potential for enhancing C2+ selectivity while maintaining high stability of nanocatalysts for CO2 reduction to liquid fuels.
用于电化学二氧化碳还原的钯纳米催化剂上原子层铜的外延生长
二氧化碳还原反应(CO2RR)有助于燃料和化学品的可持续合成。虽然铜(Cu)可以实现 CO2 到多碳产物(C2+)的转化,但由于铜原子在反应条件下的高度动态性质,铜基电催化剂(尤其是纳米催化剂)面临着选择性差和稳定性低的挑战。核壳结构以原子级的精度调节铜覆盖层与基底的相互作用,为解决这些问题提供了一种很有前景的方法。在此,我们报告了 Pd@Cu 核壳结构,该结构在具有 {100} 面的单晶 Pd 纳米管上具有原子级厚度和纳米级厚度的铜覆盖层,可促进 CO2 到 C2+ 的转化。我们通过原子尺度扫描透射电子显微镜(STEM)成像和电子能量损失光谱(EELS)研究了原子尖锐的钯/铜界面的微观结构和表面成分。我们的研究结果表明,原子层铜在钯上外延生长并适应钯基底的晶格。由于铜与钯之间的强相互作用,原子层铜在钯上的反应驱动迁移被有效抑制。虽然 Pd 只能将 CO2 还原成 C1 产物,但 Pd 上的原子层 Cu 却能在 CO2RR 过程中启动 C2+ 生成。Pd 上的厚铜覆盖层(∼15 nm)进一步提高了 C2+ 的远达效率,同时经历了显著的结构重构,只有靠近 Pd 表面的 2-3 nm 铜层在 CO2RR 之后保持稳定并能抵抗铜迁移。我们预计,具有中等铜壳厚度的 Pd@Cu 核壳结构在提高 C2+ 选择性方面具有巨大潜力,同时还能保持用于将 CO2 还原成液体燃料的纳米催化剂的高稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


