Elemental Sulfur/Selenium-Mediated Metal-Free Phosphinothioation and Phosphinoselenoation of Vinylsulfonium Salts with P–H Bonds
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
An efficient and facile method has been developed for the construction of novel P–S–C and P–Se–C bonds by facilitating the three-component cross-coupling reaction of P–H bonds with elemental sulfur/selenium and vinylsulfonium salts, utilizing sodium bicarbonate as a base. This approach eliminates the need for the use of toxic and odorous active sulfur/selenium reagents and noble metals, thereby offering a new pathway for synthesizing S-phosphinothioates and Se-phosphinoselenoates via the organic conversion of inorganic sources. The reaction has showcased remarkable versatility in terms of substrate applicability, particularly for organophosphorus compounds containing P–H bonds and vinylsulfonium salt derivatives. The resulting phosphinothioation/phosphinoselenoation products can be obtained with high yield and regioselectivity. Additionally, a plausible reaction mechanism for this transformation has been proposed based on step-by-step control experiments and 31P NMR tracking analysis.
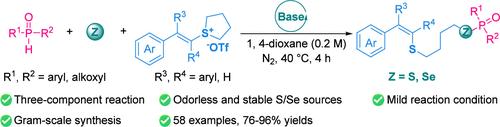
元素硫/硒介导的具有 P-H 键的乙烯基锍盐的无金属硫代磷酸化和硒代磷酸化反应
利用碳酸氢钠作为碱,通过促进 P-H 键与元素硫/硒和乙烯基锍盐的三组分交叉偶联反应,开发出了一种高效简便的方法,用于构建新型 P-S-C 和 P-Se-C 键。这种方法无需使用有毒、有味的活性硫/硒试剂和贵金属,从而为通过无机源的有机转化合成 S-硫代磷酸酯和 Se-膦酰硒酸酯提供了一条新途径。就底物的适用性而言,该反应具有显著的多功能性,尤其适用于含有 P-H 键的有机磷化合物和乙烯基锍盐衍生物。由此产生的硫代磷酸化/膦硒化产物具有高产率和区域选择性。此外,根据逐步控制实验和 31P NMR 跟踪分析,还提出了这一转化的合理反应机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


