Solvent Effects and Internal Functions Control Molecular Recognition of Neutral Substrates in Functionalized Self-Assembled Cages
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
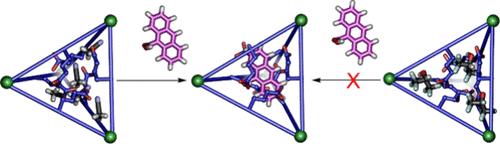
A suite of internally functionalized Fe4L6 cage complexes has been synthesized with lipophilic end groups to allow dissolution in varied solvent mixtures, and the scope of their molecular recognition of a series of neutral, nonpolar guests has been analyzed. The lipophilic end groups confer cage solubility in solvents with a wide range of polarities, from hexafluoroisopropanol (HFIP) to tetrahydrofuran, and the hosts show micromolar affinities for neutral guests, despite having no flat panels enclosing the cavity. These hosts allow interrogation of the effects of an internal functional group on guest binding properties, as well as solvent-based driving forces for recognition. Introducing polar effects to the interior of the cavity enhances guest binding affinity in nonpolar solvents; adding space-filling aliphatic groups reduces affinity in all cases. While high dielectric solvents such as acetonitrile strongly favor guest binding, “low dielectric, high polarity” solvents such as HFIP strongly occupy the cavity and prevent guest recognition. Analysis of the cage optical transitions shows that the guests interact with the central ligand cores and reside in close proximity to the internal functions. These results have implications for supramolecular catalysis: balancing directed host:guest interactions (e.g., H-bonds) with entropic effects from solvent displacement is essential for reactions in these (and related) biomimetic hosts.
溶剂效应和内部功能控制中性底物在功能化自组装笼中的分子识别
我们合成了一系列内部官能化的 Fe4L6 笼状配合物,这些配合物带有亲油末端基团,可以溶解在不同的混合溶剂中,我们还分析了这些配合物对一系列中性、非极性客体的分子识别范围。亲脂性末端基团赋予了这些宿主在从六氟异丙醇(HFIP)到四氢呋喃等多种极性溶剂中的笼溶性,而且尽管没有平板包围空腔,这些宿主对中性客体仍表现出微摩级的亲和力。通过这些宿主,可以研究内部官能团对客体结合特性的影响,以及基于溶剂的识别驱动力。在空腔内部引入极性效应,可增强客体在非极性溶剂中的结合亲和力;在所有情况下,加入填充空间的脂肪族基团都会降低亲和力。乙腈等高介电常数溶剂非常有利于客体结合,而 HFIP 等 "低介电常数、高极性 "溶剂则会强烈占据空腔,阻止客体识别。对笼状光学转变的分析表明,客体与中心配体核相互作用,并靠近内部功能。这些结果对超分子催化具有重要意义:平衡有方向性的主:客体相互作用(如 H 键)与溶剂置换产生的熵效应,对这些(及相关)仿生主体内的反应至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


