Zn(II)-Stabilized Azo-Anion Radical-Catalyzed Dehydrogenative Synthesis of Olefins
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, we describe a Zn-catalyzed atom-economical, inexpensive, and sustainable method for preparing a broad spectrum of substituted olefins utilizing alcohols as the main precursor. Using a Zn(II) complex [ZnLCl2] (1) of the redox-noninnocent ligand 2-((4-chlorophenyl)diazenyl)-1,10-phenanthroline (L), various (E)-olefins were prepared in good yields by coupling alcohols with sulfones and aryl cyanides under an inert atmosphere. Under an aerial atmosphere, vinyl nitriles were isolated in up to 82% yield reacting alcohols with benzyl cyanides in the presence of 1. Control experiments and mechanistic investigation indicate the active involvement of the aryl-azo ligand as an electron and hydrogen reservoir, permitting 1 to perform as a promising catalyst.
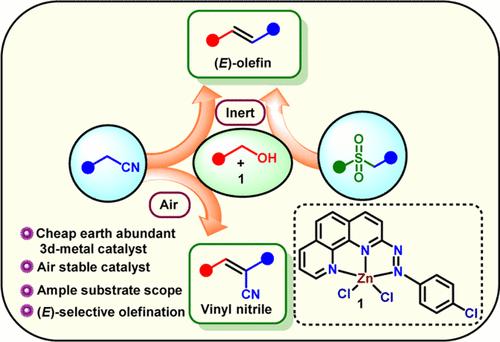
在此,我们介绍了一种锌催化的原子经济、廉价且可持续的方法,该方法利用醇作为主要前体,制备了多种取代烯烃。利用氧化还原非无效配体 2-((4-氯苯基)偶氮)-1,10-菲罗啉(L)的 Zn(II) 复合物 [ZnLCl2] (1),通过在惰性气氛下将醇与砜和芳基氰化物偶联,以良好的产率制备了各种 (E)- 烯烃。对照实验和机理研究表明,芳基偶氮配体作为电子和氢库的积极参与,使 1 成为一种前景看好的催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


