Unexpected Reaction of Dialkyl α-Hydroxy-benzylphosphonates with Dialkyl Phosphites and a Few Related Reactions
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
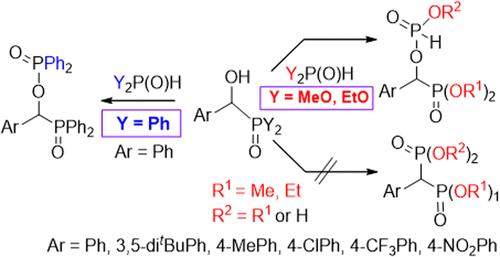
The condensation of dialkyl α-hydroxy-benzylphosphonates with dialkyl phosphites and that of α-hydroxybenzyl-diphenylphosphine oxide with diphenylphosphine oxide unexpectedly gave the corresponding phosphorylated α-hydroxy derivatives. This new reaction proved to be general. The formation of the two products may be similar and involves the attack of the hydroxy group of the α-hydroxyphosphonate or α-hydroxyphosphine oxide on the phosphorus atom of the trivalent tautomer form (Y2POH) of the Y2P(O)H reagent (Y= MeO, EtO, or Ph) going with the elimination of an alcohol and water molecule, respectively. The mechanism was supported by DFT computations at the M062X/6-31G (d,p) level of theory, including suitable proton transfer networks. The condensations discussed are typical autocatalytic reactions promoted by the alcohol or water molecules formed. The initial promoters are the traces of water inevitably present in the mixture. In the reaction of α-hydroxyphosphonates with dialkyl phosphites, the −P(O)(OR)H derivative is the primary product that is partially hydrolyzed to the −P(O)(OH)H species by the traces of water under the conditions of the reaction. Arbuzov reaction of diethyl α-bromobenzylphosphonate with ethyl diphenyphosphinite afforded the target-like phenylmethylene-phosphine oxide─phosphonate derivative.
二烷基α-羟基苄基膦酸盐与二烷基亚磷酸盐的意外反应及一些相关反应
α-羟基苄基膦酸二烷基酯与亚磷酸二烷基酯的缩合,以及α-羟基苄基二苯基氧化膦与二苯基氧化膦的缩合,意外地得到了相应的磷酸化α-羟基衍生物。事实证明,这种新反应具有普遍性。这两种产物的形成过程可能相似,都是 α-羟基膦酸盐或 α-羟基氧化膦的羟基攻击 Y2P(O)H 试剂(Y= MeO、EtO 或 Ph)的三价同分异构体形式(Y2POH)的磷原子,同时分别消除一个醇分子和一个水分子。该机理得到了 M062X/6-31G (d,p) 理论水平的 DFT 计算的支持,包括合适的质子转移网络。所讨论的缩合反应是由所形成的醇分子或水分子促进的典型自催化反应。最初的促进剂是混合物中不可避免存在的微量水。在 α-羟基膦酸盐与二烷基亚磷酸盐的反应中,-P(O)(OR)H 衍生物是主要产物,在反应条件下会被微量水部分水解为-P(O)(OH)H 物种。α-溴苄基膦酸二乙酯与二苯基膦酸乙酯的 Arbuzov 反应生成了类似目标的苯基亚甲基氧化膦─膦酸盐衍生物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


