Programmable Morphology-Adaptive Peptide Nanoassembly for Enhanced Catalytic Therapy
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
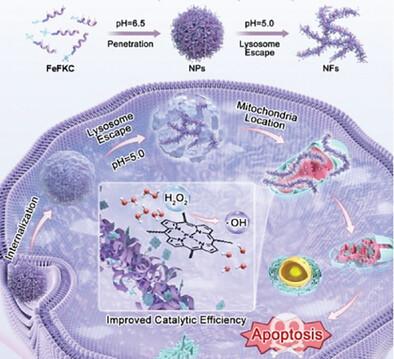
Nanocatalytic therapy holds significant promise in cancer treatment by exploiting the high oxidative stress within tumor cells. However, efficiently delivering nanocatalytic agents to tumor tissues and maximizing their catalytic activity in situ remain critical challenges. Morphology-adaptive delivery systems, capable of adjusting their physical form in response to physiological conditions, offer unique spatiotemporal control for navigating complex biological environments like the tumor microenvironment. While designing systems that undergo multiple shape transformations often involves complex stimuli-responsive mechanisms, making programmable responses through simple designs highly desirable yet challenging. Here, FeFKC, an innovative adaptive material is introduced that achieves multi-step morphological transformations at the tissue level and amplifies catalytic activity through a straightforward design. As the microenvironmental pH decreases during drug delivery, FeFKC dynamically transitions between single chains, nanoparticles, and nanofibers. This programmable shape-shifting facilitates deep tumor penetration, enhanced cellular uptake, and lysosomal escape, significantly improving its catalytic efficiency in nanocatalytic tumor therapy. In vivo studies demonstrate that FeFKC achieves impressive tumor suppression efficacy of up to 95% without notable biosafety concerns. The findings highlight the potential of adaptive nanomaterials with programmable shape-transforming capabilities to overcome biological barriers and enhance catalytic therapy, opening new avenues for cancer treatment and other complex diseases.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


