New promoters based on amino acids modified with nitrilotriacetic acid for efficient storage of methane as gas hydrates without foaming
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Solidified natural gas (SNG) is a perspective method for transportation and storing hydrocarbon gases under mild conditions. The aim of this work is to synthesize new compounds based on several amino acids (leucine, methionine, phenylalanine, norleucine, aspartic acid, valine, glutamic acid, norvaline, proline, threonine, alanine, 6-aminohexanoic acid, cysteic acid) with nitrilotriacetic acid (NTA + AA) to improve the efficiency of methane transition from gas phase into hydrate form and compare them with to pure amino acids and sodium dodecyl sulfate (SDS) as well known gas hydrate promoters. According to the results of high-pressure autoclave experiments, 7 NTA + AA samples out of 13 increase methane uptake and water to hydrate conversion better than SDS, which is a well-known effective promoter of hydrate formation, and 12 NTA + AA samples out of 13 have shorter induction times than SDS. In addition, visual observations showed that all the novel promoters synthesized from amino acids and nitrilotriacetic acid do not cause foaming throughout the process of methane hydrate dissociation, which improves the performance of this type of promoter in comparison with SDS. The efficiency of kinetic hydrate promoters at 0.05 wt% concentration by maximum mole consumption of methane decreased in the range of samples: NTA + Phenylalanine > NTA + Glutamic acid > NTA + Leucine > NTA + Norleucine > NTA + Aspartic acid > NTA + Alanine > NTA + Valine > NTA + Norvaline > SDS > NTA + Proline > NTA + Methionine > NTA + Threonine > NTA + Cysteic acid > NTA + 6-aminohexanoic acid. NTA + Phe (nitrilotriacetic acid + phenylalanine) was able to achieve 98.2 % water to hydrate conversion (0.164 mole gas/mole water) at 0.05 wt% concentration, and its induction time was independent of concentration and approximately equal to 30 min, which was about half that of SDS and pure phenylalanine. Thus NTA + Phe can be considered as one of the best promoters of methane gas hydrate formation, which can be used at low concentrations. Modification of amino acids with nitrilotriacetic acid was shown to significantly improve the efficiency of the compounds as kinetic promoters of hydrate formation, both in terms of induction time and methane uptake, compared to pure amino acids, which makes it possible to create more effective eco-friendly non-foaming promoters of methane hydrate formation used at low concentrations.
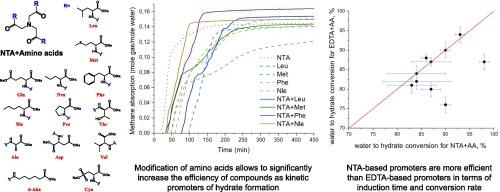
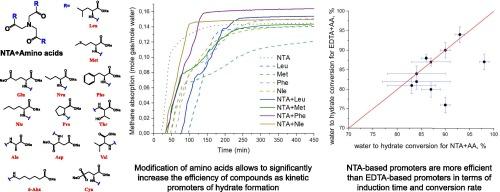
以硝基三乙酸修饰的氨基酸为基础的新型促进剂,用于甲烷作为天然气水合物的高效储存而不起泡
固化天然气(SNG)是一种在温和条件下运输和储存烃类气体的理想方法。本研究的目的是用硝酸三乙酸(NTA + AA)合成几种氨基酸(亮氨酸、蛋氨酸、苯丙氨酸、去甲亮氨酸、天冬氨酸、缬氨酸、谷氨酸、正缬氨酸、脯氨酸、苏氨酸、丙氨酸、6-氨基己酸、半胱酸)的衍生物,与纯氨基酸和十二烷基硫酸钠(SDS)相比,提高甲烷从气相转化为水合物的效率。高压高压灭菌实验结果表明,13个样品中有7个NTA + AA样品的甲烷吸收率和水成水合物的转化率优于SDS, SDS是众所周知的水合物形成的有效促进剂,13个样品中有12个NTA + AA样品的诱导时间比SDS短。此外,目测结果表明,所有由氨基酸和硝基三乙酸合成的新型启动子在甲烷水合物解离过程中都不会产生泡沫,与SDS相比,这类启动子的性能得到了提高。动力学水合物促进剂的效率在0.05 wt %由最多消耗甲烷的摩尔浓度减少样本的范围:NTA + 苯丙氨酸 祝辞 NTA + 谷氨酸 祝辞 NTA + 亮氨酸 祝辞 NTA + 正亮氨酸 祝辞 NTA + 天冬氨酸 祝辞 NTA + 丙氨酸 祝辞 NTA + 缬氨酸 祝辞 NTA + 戊氨酸 祝辞 SDS祝辞NTA + 脯氨酸 祝辞 NTA + 蛋氨酸 祝辞 NTA + 苏氨酸 祝辞 NTA + 半胱氨酸 祝辞 NTA + 6-aminohexanoic酸。NTA + Phe (nitrilotriacetic acid + phenylalanine)在0.05 wt%的浓度下能达到98.2% %的水水合物转化率(0.164 mol gas/mol water),其诱导时间与浓度无关,约为30 min,约为SDS和纯苯丙氨酸的一半。因此,NTA + Phe可以被认为是甲烷天然气水合物形成的最佳促进剂之一,可以在低浓度下使用。与纯氨基酸相比,用硝基三乙酸修饰氨基酸可以显著提高化合物作为水合物形成动力学促进剂的效率,无论是在诱导时间还是在甲烷吸收方面,这使得在低浓度下使用更有效的生态友好型非发泡甲烷水合物促进剂成为可能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


