Not Cutting Corners: Bioderived Triggers Driving Oxidative Main Chain Scission of Poly(ethylene terephthalate)
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
About 20–34 billion poly(ethylene terephthalate) (PET) bottles from the beverage industry leak into aquatic ecosystems annually, necessitating the development of urgent strategies to treat waterborne plastic pollution. Inspired by the scalability of water disinfection infrastructure and protocols, we present a dual depolymerization approach relying on oxidation, followed by hydrolysis. Incorporating bioderived monounsaturated C18 diacid (C18:1-DA) counits at low dosages (2–5%) in the PET backbone overcomes the diffusional limitations of depolymerizing PET in the solid state by suppressing the glass transition temperature of the copolymer by 20 °C. Cryomilled C18:1-PET powder suspended in an oxidant-loaded alkaline slurry underwent bulk depolymerization to oligomers at 80–100 °C via oxidative scissions at the internally located unsaturations. In contrast, conventional PET undergoes only minor end-chain scission under mild alkaline conditions. These oligomers are suitable for low-energy repolymerization or facile solvolysis to monomers. A permanganate-periodate oxidant couple demonstrated successful oxidation through the bulk of the polymer, which subsequently was hydrolyzed to monomers. This model system serves as a proxy for ozonolysis, followed by mild hydrolysis to reduce the energetics of alkaline hydrolysis. This integrated oxidation–hydrolysis strategy paves the way for the industrial adoption of cleaner, advanced oxidation processes, such as ozonolysis for plastic pretreatment, further enabling commercialized chemical recycling of unsaturation-containing polyesters.
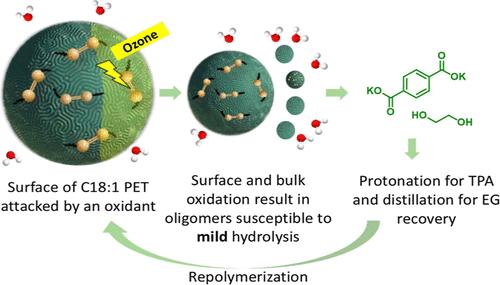
不走弯路:驱动聚对苯二甲酸乙二醇酯氧化主链裂解的生物触发器
每年约有 200-340 亿个来自饮料行业的聚对苯二甲酸乙二酯(PET)瓶泄漏到水生生态系统中,因此有必要制定紧急战略来处理水生塑料污染。受水消毒基础设施和方案可扩展性的启发,我们提出了一种先氧化后水解的双重解聚方法。在 PET 骨架中加入低剂量(2-5%)的生物源单不饱和 C18 二酸(C18:1-DA)偶联剂,通过将共聚物的玻璃化转变温度降低 20 °C,克服了固态 PET 解聚的扩散限制。悬浮在含氧化剂碱性浆料中的冷冻C18:1-PET粉末在80-100 °C温度下,通过内部不饱和层的氧化裂解,大量解聚成低聚物。相比之下,传统 PET 在温和的碱性条件下仅会发生轻微的端链裂解。这些低聚物适用于低能再聚合或容易溶解为单体。高锰酸盐-碘酸氧化剂偶联物成功地氧化了聚合物的大部分,随后水解为单体。该模型系统可作为臭氧分解的替代物,随后进行温和水解,以降低碱性水解的能耗。这种综合氧化-水解策略为工业上采用更清洁的高级氧化工艺(如用于塑料预处理的臭氧分解)铺平了道路,从而进一步实现了含不饱和聚酯的商业化化学回收。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


