Efficient Separation of Pr(III) and Nd(III) via Shear-Induced Dissociation Coupling with Ultrafiltration: Insights from Experiments and Theory
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The separation of Pr(III) and Nd(III) poses a significant challenge due to their similar physicochemical properties, including comparable ionic radii and coordination chemistry. In this study, acidic phosphoric chitosan (aPCS) was employed as a complexing agent in the shear-induced dissociation coupling with ultrafiltration (SID-UF) technique for the sequential separation of Pr(III) and Nd(III). SID-UF uses shear stress to reduce membrane contamination and enables efficient metal ion separation while allowing complexing agent recovery without acid decomplexation, simplifying the process and lowering costs. The complexation kinetics of Pr(III) and Nd(III) with aPCS were investigated for the first time, following a pseudo-first-order model. The SID-UF technique achieved over 97% removal efficiency under optimized conditions, with a separation factor βPr/Nd of 12.01. DFT calculations showed greater electron transfer for Nd(III) (0.2218 e) compared to Pr(III) (0.2149 e), indicating stronger complexation with Nd(III). The interaction energies further confirmed this, with Nd(III) exhibiting a more favorable binding energy of −32.19 kcal mol–1 compared to −31.09 kcal mol–1 for Pr(III). These results highlight the high selectivity, efficiency, and environmental benefits of SID-UF, making it a promising alternative for industrial rare-earth separations.
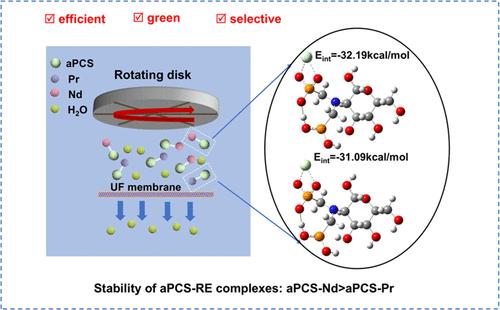
剪切诱导离解耦合超滤高效分离Pr(III)和Nd(III):来自实验和理论的见解
由于镨(III)和钕(III)具有相似的物理化学性质,包括可比的离子半径和配位化学性质,因此它们的分离是一项重大挑战。在本研究中,酸性磷酸壳聚糖(aPCS)被用作剪切力诱导解离耦合超滤(SID-UF)技术中的络合剂,用于依次分离 Pr(III)和 Nd(III)。SID-UF 利用剪切应力减少膜污染,实现高效的金属离子分离,同时无需酸解络合即可回收络合剂,从而简化了工艺并降低了成本。根据伪一阶模型,首次研究了 Pr(III) 和 Nd(III) 与 aPCS 的络合动力学。在优化条件下,SID-UF 技术的去除效率超过 97%,分离因子 βPr/Nd 为 12.01。DFT 计算显示,与 Pr(III) (0.2149 e) 相比,Nd(III) (0.2218 e) 的电子转移更大,表明与 Nd(III) 的络合更强。相互作用能进一步证实了这一点,Nd(III) 的结合能为 -32.19 kcal mol-1,而 Pr(III) 的结合能为 -31.09 kcal mol-1。这些结果凸显了 SID-UF 的高选择性、高效率和环境效益,使其成为工业稀土分离的一种有前途的替代方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


