Ca2+/Si4+ Modification of the (Gd,Lu)AG Garnet for Enhanced Broadband Cr3+ Luminescence of High Thermal Stability
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
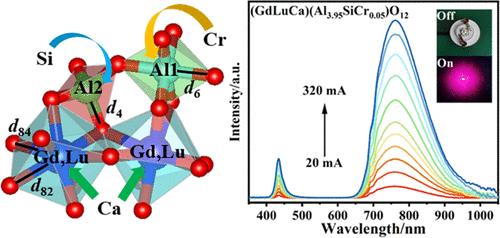
Near-infrared (NIR)-emitting phosphors with high quantum efficiency and thermal stability are crucial to NIR pc-LEDs. Garnet-structured (GdLuCa)(Al4–zSiCrz)O12 (z = 0.01–0.2) and (Gd2–xLuCax)(Al4.95–xSixCr0.05)O12 (x = 0.2–1.0) new phosphors with promising NIR luminescence under blue light excitation were designed and fabricated by a solid-state reaction in this work. It was analyzed that the Ca2+, Cr3+, and Si4+ ions would replace Gd3+ in [GdO8], Al1 in [Al1O6], and Al2 in [Al2O4], respectively, and the optimal Cr3+ content is z = 0.05, above which concentration quenching would occur via an electric dipole–dipole interaction. Increasing Ca2+/Si4+ substitution up to x = 1.0 led to luminescence enhancement by a factor of up to 1.85 and internal/external quantum efficiency (%) increment from ∼25.9/10.7 to 63.4/27.5, and all of the phosphors showed excellent thermal stability (I423 K/I298 K ≥ 87.6%). The luminescence properties of Cr3+ were discussed in detail through systematic investigation of the effects of Cr3+ and Ca2+/Si4+ contents on the crystal structure, local coordination, and crystal field. With the NIR pc-LED device integrated from the optimal phosphor (x = 1.0) and a blue LED chip, electroluminescence manifested potential applications in night vision and medical diagnosis.
(Gd,Lu)AG石榴石的Ca2+/Si4+改性增强高热稳定性宽带Cr3+发光
具有高量子效率和热稳定性的近红外发光荧光粉对近红外pc- led至关重要。本文采用固相反应设计制备了石榴石结构(GdLuCa)(Al4-zSiCrz)O12 (z = 0.01-0.2)和(Gd2-xLuCax)(Al4.95-xSixCr0.05)O12 (x = 0.2-1.0)蓝光激发下具有近红外发光前景的新型荧光粉。分析表明,[GdO8]中的Gd3+、[al106]中的Al1、[Al2O4]中的Al2将分别被Ca2+、Cr3+、Si4+离子取代,且最佳的Cr3+含量为z = 0.05,在此值以上,通过电偶极子-偶极子相互作用发生浓度猝灭。当Ca2+/Si4+取代量增加到x = 1.0时,发光增强系数高达1.85,内/外量子效率(%)从~ 25.9/10.7增加到63.4/27.5,所有荧光粉都表现出优异的热稳定性(I423 K/I298 K≥87.6%)。通过系统研究Cr3+和Ca2+/Si4+含量对晶体结构、局部配位和晶体场的影响,详细讨论了Cr3+的发光特性。利用最佳荧光粉(x = 1.0)和蓝色LED芯片集成的近红外pc-LED器件,电致发光在夜视和医疗诊断方面显示出潜在的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
文献相关原料
公司名称
产品信息
阿拉丁
SiO2
阿拉丁
CaCO3
阿拉丁
Al2O3
阿拉丁
Cr2O3
阿拉丁
SiO2
阿拉丁
CaCO3
阿拉丁
Al2O3
阿拉丁
Cr2O3
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


