Rap1 and mTOR signaling pathways drive opposing immunotoxic effects of structurally similar aryl-OPFRs, TPHP and TOCP
IF 9.7
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Aryl organophosphorus flame retardants (aryl-OPFRs), commonly used product additives with close ties to daily life, have been regrettably characterized by multiple well-defined toxicity risks. Triphenyl phosphate (TPHP) and tri-o-cresyl phosphate (TOCP), two structurally similar aryl-OPFRs, were observed in our previous study to exhibit contrasting immunotoxic effects on THP-1 macrophages, yet the underlying mechanisms remain unclear. This study sought to address the knowledge gap by integrating transcriptomic and metabolomic analyses to elucidate the intricate mechanisms. During individual omics analyses, we unfortunately only obtained highly similar results for both TPHP and TOCP, failing to identify the key reasons for their differences. These results revealed comparable disturbances induced by both compounds, including disruptions in nucleic acid synthesis and energy metabolism, blocking ADP to ATP conversion by reducing TCA cycle intermediates, consequently leading to ATP depletion. However, through integrative analysis, specific pathways affected by each compound were successfully identified, shedding light on their unique effects. TPHP reduced GTP levels necessary for Rap1 activation, thereby inhibiting phagocytosis and adhesion of THP-1 macrophages. Conversely, TOCP stimulated the mTOR signaling pathway, enhancing phosphorylation of downstream proteins S6K, RHOA, and PKC, consequently promoting immune responses. This study not only clarified the distinct immunotoxic mechanisms of TPHP and TOCP but also provided critical insights into how structural variations in aryl-OPFRs can lead to markedly different immune responses, thereby informing future risk assessments and regulatory strategies for these compounds.
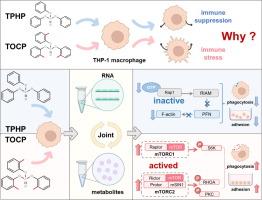

Rap1和mTOR信号通路驱动结构相似的芳基-OPFR(TPHP和TOCP)产生截然相反的免疫毒性效应
芳基有机磷阻燃剂(arryl-OPFRs)是与日常生活密切相关的常用产品添加剂,但令人遗憾的是,它们具有多种明确的毒性风险。磷酸三苯酯(TPHP)和磷酸三邻甲酚酯(TOCP)是两种结构相似的芳基阻燃剂,我们之前的研究观察到它们对 THP-1 巨噬细胞表现出截然不同的免疫毒性作用,但其潜在机制仍不清楚。本研究试图通过整合转录组学和代谢组学分析来阐明其复杂的机制,从而填补这一知识空白。遗憾的是,在对 TPHP 和 TOCP 进行单独的全局分析时,我们只得到了高度相似的结果,未能找出造成它们之间差异的关键原因。这些结果揭示了两种化合物诱发的相似干扰,包括破坏核酸合成和能量代谢,通过减少 TCA 循环中间产物阻断 ADP 到 ATP 的转换,从而导致 ATP 消耗。不过,通过综合分析,成功确定了受每种化合物影响的特定途径,从而揭示了它们的独特作用。TPHP 降低了 Rap1 激活所需的 GTP 水平,从而抑制了 THP-1 巨噬细胞的吞噬和粘附。相反,TOCP 会刺激 mTOR 信号通路,增强下游蛋白 S6K、RHOA 和 PKC 的磷酸化,从而促进免疫反应。这项研究不仅阐明了 TPHP 和 TOCP 不同的免疫毒性机制,还提供了关于芳基-OPFR 结构变异如何导致明显不同的免疫反应的重要见解,从而为这些化合物未来的风险评估和监管策略提供了信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
文献相关原料
公司名称
产品信息
阿拉丁
methanol
阿拉丁
isopropanol
阿拉丁
HPLC-grade methanol
阿拉丁
isopropanol
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


