Chemical engineering of CRISPR–Cas systems for therapeutic application
IF 122.7
1区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Clustered regularly interspaced short palindromic repeats (CRISPR) technology has transformed molecular biology and the future of gene-targeted therapeutics. CRISPR systems comprise a CRISPR-associated (Cas) endonuclease and a guide RNA (gRNA) that can be programmed to guide sequence-specific binding, cleavage, or modification of complementary DNA or RNA. However, the application of CRISPR-based therapeutics is challenged by factors such as molecular size, prokaryotic or phage origins, and an essential gRNA cofactor requirement, which impact efficacy, delivery and safety. This Review focuses on chemical modification and engineering approaches for gRNAs to enhance or enable CRISPR-based therapeutics, emphasizing Cas9 and Cas12a as therapeutic paradigms. Issues that chemically modified gRNAs seek to address, including drug delivery, physiological stability, editing efficiency and off-target effects, as well as challenges that remain, are discussed. CRISPR technology is revolutionizing the development of therapies for genetic disorders. However, the application of CRISPR-based therapeutics is challenged by factors impacting stability, efficiency, delivery and safety. This Review focuses on chemical engineering of CRISPR–Cas systems to address these issues, it assesses next-generation CRISPR–Cas systems, and it highlights CRISPR-based therapies that are approved or in clinical development.

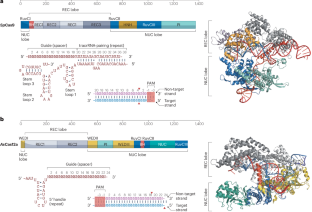
CRISPR-Cas系统的化学工程治疗应用
成簇间隔短回文重复序列(CRISPR)技术改变了分子生物学和基因靶向治疗的未来。CRISPR 系统由 CRISPR 相关(Cas)内切酶和引导 RNA(gRNA)组成,可通过编程引导序列特异性结合、切割或修饰互补 DNA 或 RNA。然而,基于 CRISPR 的疗法的应用受到一些因素的挑战,如分子大小、原核或噬菌体起源以及对 gRNA 辅助因子的基本要求,这些因素都会影响疗效、递送和安全性。本综述重点关注 gRNA 的化学修饰和工程方法,以增强或实现基于 CRISPR 的疗法,并强调 Cas9 和 Cas12a 作为治疗范例。文中讨论了化学修饰的 gRNA 试图解决的问题,包括药物输送、生理稳定性、编辑效率和脱靶效应,以及仍然存在的挑战。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews. Drug Discovery
医学-生物工程与应用微生物
CiteScore
137.40
自引率
0.30%
发文量
227
期刊介绍:
Nature Reviews Drug Discovery is a monthly journal aimed at everyone working in the drug discovery and development arena.
Each issue includes:
Highest-quality reviews and perspectives covering a broad scope.
News stories investigating the hottest topics in drug discovery.
Timely summaries of key primary research papers.
Concise updates on the latest advances in areas such as new drug approvals, patent law, and emerging industry trends and strategies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


