Unlocking Charge Transfer Limitation toward Advanced Low-Temperature Sodium-Ion Batteries
IF 19.3
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Sodium-ion batteries (SIBs) are recognized as promising large-scale energy storage systems but suffer from sluggish kinetics at low temperatures. Herein, we proposed a carbon nanotubes-modified P2-Na0.67Mn0.67Ni0.33O2 (NMNO-CNTs) cathode and tetrahydrofuran (THF)-containing dimethyl-based electrolyte to unlock the charge transfer limitation of SIBs at low temperatures. A highly conductive network constructed by CNTs ensures fast surface electron transfer. The introduction of THF enables an anion-rich solvation structure, which facilitates the formation of a robust NaF-rich electrode–electrolyte interface with accelerated desolvation and uniform Na deposition. As a result, the Na||NMNO-CNTs cell delivers a capacity of 83.4 mAh g–1 even after 3600 cycles with a decay rate of 0.002% per cycle at −40 °C. More importantly, the hard carbon||NMNO-CNTs full cell exhibits an energy density of 237.6 Wh kg–1 with 86.5% retention after 1500 cycles at −40 °C. The work highlights the key role of charge transfer kinetics for advanced low-temperature SIBs.
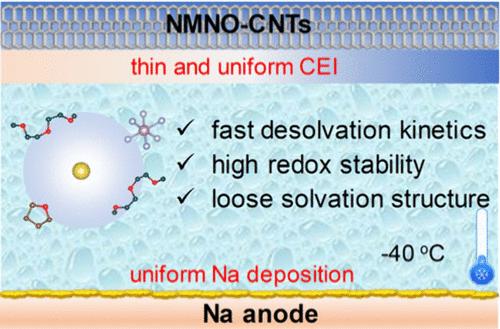
打破电荷转移限制,开发先进的低温钠离子电池
钠离子电池(SIBs)是公认的前景广阔的大规模储能系统,但在低温条件下存在动力学缓慢的问题。在此,我们提出了一种碳纳米管修饰的 P2-Na0.67Mn0.67Ni0.33O2 (NMNO-CNTs)阴极和含二甲基的四氢呋喃(THF)电解质,以解开钠离子电池在低温下的电荷转移限制。由碳纳米管构建的高导电性网络可确保快速的表面电子转移。四氢呋喃的引入实现了富含阴离子的溶解结构,有利于形成稳固的富含 NaF 的电解质-电解质界面,从而加速解溶解和均匀的 Na 沉积。因此,Na||NMNO-CNTs 电池在 -40 °C 下经过 3600 个循环后仍能提供 83.4 mAh g-1 的容量,每个循环的衰减率为 0.002%。更重要的是,硬碳||NMNO-CNTs全电池在-40 °C下循环1500次后,能量密度达到237.6 Wh kg-1,保持率为86.5%。这项工作凸显了电荷转移动力学在先进的低温 SIB 中的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


