The Detail Matters: Unveiling Overlooked Parameters in the Mechanochemical Synthesis of Solid Electrolytes
IF 19.3
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
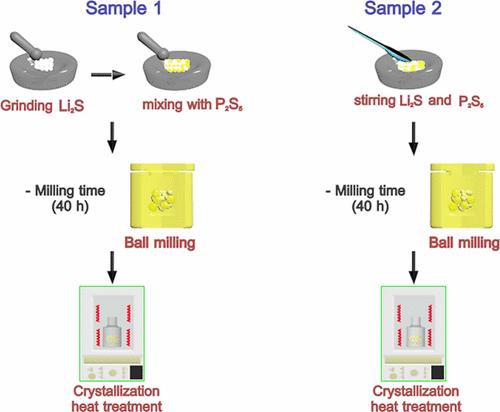
The advent of all-solid-state lithium-ion batteries has advanced energy storage technologies with the development of highly conductive solid electrolytes. Numerous researchers have reported the structural and electrochemical performance of solid electrolytes obtained through different production techniques and with different compositions. (1,2) However, even in relatively robust production techniques using ball-milling with the same composition and stoichiometry, only a minute difference in the synthesis process can significantly affect the crystallization mechanisms and resulting ionic conductivity, thereby highlighting the importance of overlooked parameters. This Viewpoint demonstrates the effects of “premixing”─mixing the precursors with a mortar and pestle prior to the mechanochemical synthesis of glassy solid electrolytes, particularly Li2S–P2S5 sulfides and the newly emerging NaTaCl6 halides─on the structure and transport of the resulting products. Crystal structures and amorphous configurations of sulfide and chloride electrolytes with high ionic conductivities and excellent mechanical properties have been identified. (3−11) These electrolytes are commonly produced through mechanochemical synthesis using ball-milling, which has been widely utilized with various chemical compounds. (12−14) Li7P3S11 is recognized as a metastable phase that is nucleated by the subsequent heat treatment of Li2S–P2S5 glasses produced by planetary ball-milling. NaTaCl6 is recognized as a mixture of crystal and amorphous phases that shows an excellent electrochemical window. In both cases, a wide range of ball-milling experimental parameters have been investigated, including the amount of powder, number of balls, rotation speed, and ball-milling time, leading to the successful synthesis of the target phases. (15−24) This experimental fact makes these synthesis methods for producing Li7P3S11 and NaTaCl6 seemingly very robust. Nonetheless, although the apparent crystal structures evaluated by X-ray diffraction (XRD) are almost identical, there are significant differences in their ion conductivities. (18,25−27) Moreover, only three of 15 studies reported on Li7P3S11 syntheses (3,28,29) have described the hand-mixing of starting powders prior to or intermittently during ball-milling, as given in Table S1. Similarly, one out of three studies of NaTaCl6 have described the hand-mixing of starting powders (Table S2). Although the details of the hand-mixing have been deemed to have negligible effects, this study demonstrates the importance of such a process. This Viewpoint demonstrates the impact of the unspecified details of mechanochemical synthesis on the crystallization mechanisms and ionic conductivities of the products, highlighting the importance of parameters that are often overlooked in such syntheses. Two synthesis routes based on the existing literature (3,6,17,30) were employed to synthesize Li7P3S11 using ball-milling, as shown in Scheme 1. The difference between the two routes is the implementation of a hand-mixing step prior to mechanical milling, referred to as premixing. For Sample 1, the hand-ground Li2S precursor was thoroughly mixed together with P2S5 for 20 min using an agate mortar and pestle. In contrast, the precursors of Sample 2 were just stirred with a spatula for 2 min prior to milling. Similarly, the impact of premixing on the synthesis of NaTaCl6, synthesized by mechanical milling of NaCl and TaCl5, was assessed through investigating two NaTaCl6 samples synthesized with or without premixing. The details of the compositions during the synthesis of the powders, including the required reagents and synthesis process, are given in Tables S3 and S4. Figure 1(a, b) displays the 31P magic-angle spinning nuclear magnetic resonance (31P MAS NMR) spectra of the Li7P3S11 glass produced by milling with or without premixing before heating. Despite the broad halo patterns in XRD (Figure S1), which indicate that both the samples, with and without premixing, were completely amorphous, distinctly different local anion coordination is observed in the NMR spectra. The spectra of both samples were deconvoluted into three peaks, where PS43– and P2S74– are constituent elements of the target Li7P3S11 phase and P2S64– is the local unit of the Li4P2S6 impurity phase. (19) Figure 1(c) shows a comparison of the area-size fractions of the peaks corresponding to the three anion blocks. Although a glassy phase typically contains various local structures as its nature, the amount of P2S64– in Sample 1 is less than that in Sample 2. Furthermore, the area-size ratios of P2S74–, and PS43– are 1.70 and 1.24 for Samples 1 and 2, respectively. The area-size ratio of Sample 1 approaches 2.00, which is the theoretical stoichiometric ratio of the two components in crystalline Li7P3S11, indicating the anion blocks in Sample 1 are similar to those in Li7P3S11 even before heat treatment. Data with a wider range of chemical shifts, confirming no overlap between sidebands and main peaks, are shown in Figure S2. Figure 1. 31P MAS NMR spectra and deconvoluted peak profiles of the as-milled powders of (a) Sample 1 and (b) Sample 2. (c) Area-size fractions of Samples 1 and 2. In situ synchrotron X-ray diffraction (SXRD) demonstrates evidently different crystallization processes between the two Li2S–P2S5 glasses (Figure 2). Sample 1 exhibits a single-step crystallization at approximately 220 °C. Conversely, Sample 2 undergoes a two-step crystallization process owing to the sequential formation of Li3PS4 at approximately 220 °C, followed by Li7P3S11 at approximately 240 °C. Rietveld refinement was performed on the temperature-dependent diffractograms to quantify the Li7P3S11, Li3PS4, and Li4P2S6 fractions at various temperatures. Sample 1 crystallizes into Li7P3S11 without apparent side phases, whereas Sample 2 comprises 11% Li3PS4 and 2% Li4P2S6 even at 300 °C, which can degrade its ion-transport properties. (30−33) The diffraction profiles did not show a significant difference in the peaks of the Li7P3S11 phase (Figure S3). Differential thermal analysis (DTA) confirmed the difference in the crystallization processes (Figure S4). While a sharp exothermic signal was observed in Sample 1, a broad signal with two distinct peaks was seen in Sample 2. This trend was reproducible even for the same samples in different batches, indicating the local inhomogeneity of the anion blocks in the original glass states, as revealed in the NMR data. Figure 2. SXRD data of the powders heated at 60 °C/min under a N2 flow and phase ratios from Rietveld refinement of (a) Sample 1 and (b) Sample 2. A distinctly different crystallization process induced by local structural differences leads to variations in the properties of the resulting crystalline Li7P3S11 phases. Figure S5 shows the 31P NMR spectra of the two samples after crystallization. While the quantification of local units is challenging with the spectra from the heat-treated samples due to the appearance of the cross peaks in Sample 1, the qualitative difference is clearly observed. It should be noted that such cross peaks associated with P2S74– and PS43– are commonly observed in well-crystalline Li7P3S11 phases. (34) To further investigate the differences in the crystalline phases of Samples 1 and 2, pair-distribution function (PDF) analysis was performed on Samples 1 and 2. As the data from both samples showed the appearance of long-range ordering only after heating, the two samples were largely similar. However, a slight change in G(r) in short-range order of ∼3.4 Å appeared, depending on the hand-milling processes (Figure S6). The morphologies of Samples 1 and 2 after heating showed no significant differences (Figure S7). Overall, it is evident that the premixing procedure prior to milling severely impacts the resulting structure. The above-mentioned differences led to significant differences in the ion-transport properties of the resulting materials. The powders crystallized at 280–300 °C were pressed into pellets, and their ionic conductivities were measured by temperature-dependent impedance spectroscopy to analyze the Li-ion transport in the samples. Furthermore, the migration barrier of the Li-ion conduction was determined according to the Arrhenius relation: Figure 3. Arrhenius plots of the temperature and ion conductivity of Samples 1 and 2. As another model system to assess the impact of premixing, we employed a newly found halide-based solid electrolyte, NaTaCl6, to further investigate the impact of premixing. The ion-transport properties of NaTaCl6 are reported to significantly vary with its crystallinity. (27) Moreover, the impact of elongated milling time of mechanochemical synthesis on the ion-transport properties has been revealed. (26) In this section, four samples were synthesized, either with or without premixing, and subjected to milling for either 16.5 or 100 h. Figure 4 shows the room-temperature ionic conductivity and activation energies of four samples obtained with different milling times, with or without premixing. The samples without premixing were synthesized via ball-milling; that is, the precursors were directly placed into the ball-mill cup with the milling media. Meanwhile, the samples with premixing were mixed well with a mortar and pestle before ball-milling. Prolonging the milling time from 16.5 to 100 h slightly improved the ionic conductivity. However, more significant improvements in the ionic conductivity were achieved by premixing the precursors well, whereas there is no significant difference in their XRD patterns. Notably, milling for 100 h was insufficient to achieve the same level of ion transport as that of the sample subjected to premixing. These results highlight the importance of the premixing step. Figure 4. Ionic conductivity and activation energies of mechanochemically synthesized NaTaCl6 samples under different conditions. Each sample was synthesized three times, and the error bars are the standard deviations from the three batches of samples. Li7P3S11 and NaTaCl6 syntheses highlight the crucial effect of the hand-mixing step before mechanical milling on the local structure, crystallization temperature, and ionic conductivity. Although further accumulation of experimental evidence is necessary to precisely elucidate the underlying mechanisms, the current findings are as follows: Significant variations in the fractions of the local units, indicating the local inhomogeneity, were observed without the implementation of the premixing step. These localized compositional variations can lead to diverse anionic blocks during the subsequent crystallization processes, which, in turn, alters the structure and properties of the products. In the case of NaTaCl6, extended ball-milling durations did not eliminate localized compositional variations, as inferred from the limited conductivity improvement when the ball-milling time was increased from 16.5 to 100 h. Meanwhile, the resulting ion transport was greatly improved by the introduction of a short premixing step before mechanochemical synthesis. While the impact of extended milling may vary with the chemical and mechanical properties of the precursors, the premixing procedure helps to reduce the synthesis time to obtain the target phases. Differential adhesion to the milling media was proposed as a potential cause of compositional inhomogeneity. Ball-milling homogenized mixed precursors when they were trapped between the milling media or between the media and the inner wall of the cup. This process formed powders with layered structures of various compositions. Such a reaction was promoted by mechanical mixing as the interlayer distance decreased, reducing the diffusion length required to form the target phase. However, when a heterogeneous precursor mixture was introduced into the milling cup, softer materials tended to adhere to the milling medium first. Consequently, we hypothesized that this formed thick layers with a constant diffusion length, leading to pronounced local compositional variations. Mechanical properties of starting materials and final products can be critical for proceeding the reactions. (35) As significant variation in the seemingly reproducible properties─ionic conductivity and cycling performance─is one of the big challenges in the field of solid-state batteries and attracts increasing attention, with the recent reports showcasing the critical issue, (36,37) our findings on the drastic impact of the premixing procedure before the seemingly robust mechanochemical synthesis with ball-milling further highlight the importance of the details in the synthesis conditions. The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsenergylett.4c02156. Table of the synthesis process of Li7P3S11 and NaTaCl6, characterization details, measurement results (XRD patterns, NMR spectra, DTA curves, PDF and SEM images) of Li7P3S11 (PDF) The Detail Matters: Unveiling Overlooked Parameters in the Mechanochemical Synthesis of Solid Electrolytes 1 views 0 shares 0 downloads Most electronic Supporting Information files are available without a subscription to ACS Web Editions. Such files may be downloaded by article for research use (if there is a public use license linked to the relevant article, that license may permit other uses). Permission may be obtained from ACS for other uses through requests via the RightsLink permission system: http://pubs.acs.org/page/copyright/permissions.html. A. Miura is grateful to Ms. Masae Sawamoto for preparing the powder capillaries for the SXRD measurements. The authors are grateful to Mr. Yuki Chiba at Tohoku University for his support with the solid-state MAS NMR measurements. S.O. gratefully acknowledges Toyota Riken for its financial support through the Rising Fellows Program. This study was partially supported by JST PRESTO JPMJPR21Q8, JST Gtex JPMJGX23S5, and JPMJGX23S2. This article references 37 other publications. This article has not yet been cited by other publications.
细节决定成败:揭示固体电解质机械化学合成中被忽视的参数
不同条件下机械化学合成 NaTaCl6 样品的离子电导率和活化能。每个样品合成三次,误差带为三批样品的标准偏差。Li7P3S11 和 NaTaCl6 的合成突显了机械研磨前的手工混合步骤对局部结构、结晶温度和离子导电率的关键影响。尽管需要进一步积累实验证据才能准确阐明其基本机制,但目前的研究结果如下:在没有实施预混合步骤的情况下,观察到局部单元的分数发生了显著变化,这表明了局部的不均匀性。在随后的结晶过程中,这些局部成分变化会导致不同的阴离子块,进而改变产品的结构和性质。就 NaTaCl6 而言,当球磨时间从 16.5 小时增加到 100 小时时,电导率的改善有限,由此可以推断,延长球磨时间并不能消除局部的成分变化。虽然延长研磨时间的影响可能因前驱体的化学和机械特性而异,但预混合步骤有助于缩短合成时间,从而获得目标相。有人提出,研磨介质的不同附着力是造成成分不均匀的潜在原因。当混合前驱体滞留在研磨介质之间或介质与研磨杯内壁之间时,球磨会使混合前驱体均匀化。这一过程形成了具有不同成分的分层结构的粉末。随着层间距离的减小,形成目标相所需的扩散长度缩短,机械混合促进了这种反应。然而,当异质前驱体混合物被引入研磨杯时,较软的材料往往首先附着在研磨介质上。因此,我们假设这会形成具有恒定扩散长度的厚层,从而导致明显的局部成分变化。起始材料和最终产品的机械性能对反应的进行至关重要。(35)由于看似可重现的性能--电导率和循环性能--的显著变化是固态电池领域的巨大挑战之一,并吸引了越来越多的关注,最近的报告也展示了这一关键问题,(36,37)我们的研究结果表明,在用球磨法进行看似稳健的机械化学合成之前,预混合程序会产生巨大影响,这进一步凸显了合成条件细节的重要性。辅助信息可在 https://pubs.acs.org/doi/10.1021/acsenergylett.4c02156 免费获取。Li7P3S11 和 NaTaCl6 的合成过程表、表征细节、Li7P3S11 的测量结果(XRD 图、NMR 光谱、DTA 曲线、PDF 和 SEM 图像)(PDF) The Detail Matters:揭示固体电解质机械化学合成中被忽视的参数 1 次浏览 0 次分享 0 次下载 大多数电子版辅助信息文件无需订阅 ACS Web Editions 即可获得。此类文件可按文章下载,用于研究用途(如果相关文章链接了公共使用许可,则该许可可能允许其他用途)。如需其他用途,可通过 RightsLink 许可系统 http://pubs.acs.org/page/copyright/permissions.html 向 ACS 申请许可。A. Miura 感谢 Masae Sawamoto 女士为 SXRD 测量准备了粉末毛细管。作者感谢东北大学的 Yuki Chiba 先生对固态 MAS NMR 测量的支持。S.O. 感谢丰田理研通过 Rising Fellows 计划提供的资助。本研究得到了 JST PRESTO JPMJPR21Q8、JST Gtex JPMJGX23S5 和 JPMJGX23S2 的部分支持。本文引用了 37 篇其他出版物。本文尚未被其他出版物引用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


