Cell Architecture and Dynamics of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPSC-CMs) on Hydrogels with Spatially Patterned Laminin and N-Cadherin
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
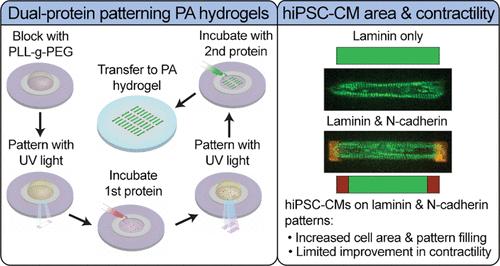
Controlling cellular shape with micropatterning extracellular matrix (ECM) proteins on hydrogels has been shown to improve the reproducibility of the cell structure, enhancing our ability to collect statistics on single-cell behaviors. Patterning methods have advanced efforts in developing human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) as a promising human model for studies of the heart structure, function, and disease. Patterned single hiPSC-CMs have exhibited phenotypes closer to mature, primary CMs across several metrics, including sarcomere alignment and contractility, area and aspect ratio, and force production. Micropatterning of hiPSC-CM pairs has shown further improvement of hiPSC-CM contractility compared to patterning single cells, suggesting that CM–CM interactions improve hiPSC-CM function. However, whether patterning single hiPSC-CMs on a protein associated with CM–CM adhesion, like N-cadherin, can drive similar enhancement of the hiPSC-CM structure and function has not been tested. To address this, we developed a novel dual-protein patterning process featuring covalent binding of proteins at the hydrogel surface to ensure robust force transfer and force sensing. The patterns comprised rectangular laminin islands for attachment across the majority of the cell area, with N-cadherin “end caps” to imitate CM–CM adherens junctions. We used this method to geometrically control single-cell CMs on deformable hydrogels suitable for traction force microscopy (TFM) to observe cellular dynamics. We seeded α-actinin::GFP-tagged hiPSC-CMs on dual-protein patterned hydrogels and verified the interaction between hiPSC-CMs and N-cadherin end caps via immunofluorescent staining. We found that hiPSC-CMs on dual-protein patterns exhibited higher cell area and contractility in the direction of sarcomere organization than those on laminin-only patterns but no difference in sarcomere organization or total force production. This work demonstrates a method for covalent patterning of multiple proteins on polyacrylamide hydrogels for mechanobiological studies. However, we conclude that N-cadherin only modestly improves single-cell patterned hiPSC-CM models and is not sufficient to elicit increases in contractility observed in hiPSC-CM pairs.
人诱导多能干细胞衍生的心肌细胞(hiPSC-CMs)在具有空间图案化层粘连蛋白和 N-粘连蛋白的水凝胶上的细胞结构和动态变化
利用水凝胶上的细胞外基质(ECM)蛋白微图案控制细胞形状已被证明可以提高细胞结构的可重复性,增强我们收集单细胞行为统计数据的能力。在人类诱导多能干细胞衍生的心肌细胞(hiPSC-CMs)作为研究心脏结构、功能和疾病的有前途的人类模型方面,图图化方法取得了进展。图图化的单hiPSC-CMs在几个指标上表现出更接近成熟的表型,包括肌节排列和收缩性、面积和纵横比以及力产生。与单细胞相比,hiPSC-CM对的微图图化进一步改善了hiPSC-CM的收缩性,这表明CM-CM相互作用改善了hiPSC-CM的功能。然而,在与CM-CM粘附相关的蛋白质(如N-cadherin)上对单个hiPSC-CM进行图图化是否可以驱动hiPSC-CM结构和功能的类似增强尚未得到测试。为了解决这个问题,我们开发了一种新的双蛋白图图化过程,该过程具有水凝胶表面蛋白质的共价结合,以确保强大的力传递和力传感。这种模式包括矩形的层粘连蛋白岛,用于附着在大部分细胞区域,n -钙粘蛋白“端帽”模仿CM-CM粘附连接。我们用这种方法在适合牵引力显微镜(TFM)观察细胞动力学的可变形水凝胶上对单细胞CMs进行几何控制。我们将α- actitin:: gfp标记的hiPSC-CMs植入双蛋白图案水凝胶上,通过免疫荧光染色验证hiPSC-CMs与N-cadherin端帽的相互作用。我们发现双蛋白模式下的hiPSC-CMs在肌节组织方向上的细胞面积和收缩性比仅层粘胶蛋白模式下的高,但在肌节组织和总力产生方面没有差异。这项工作展示了一种用于机械生物学研究的聚丙烯酰胺水凝胶上多种蛋白质的共价模式方法。然而,我们得出结论,n -钙粘蛋白仅适度改善单细胞模式的hiPSC-CM模型,不足以引起在hiPSC-CM对中观察到的收缩性增加。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


