Physical and electronic structure optimization of multivalent multi-dimensional Cu-based electrodes for efficient electrocatalytic nitrate reduction to ammonia
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The electrochemical reduction of nitrate for ammonia synthesis has attracted considerable attention due to its low energy consumption and environmental compatibility. To facilitate the industrial-scale implementation of catalysts for electrochemical ammonia production, it is crucial to consider not only the catalysts’ high catalytic activity and selectivity but also their scalable fabrication process and facile preparation methodology. This study presented a multi-dimensional composite electrode with multivalent Cu-based oxides designed using a simple immersion reduction method. Cu(OH)2 nanowires and Cu2O nanoparticles were in-situ grown on Cu foam, creating a multidimensional composite structure. Subsequently, the electrode is transformed into Cu+/Cu0 through electrochemical in-situ reduction, while the microstructure and morphology do not undergo significant changes. The electronic interactions between multivalent Cu-based oxides promoted physicochemical adsorption of NO3– molecules and optimize electron and proton transfer pathways. At a potential of −0.8 V (vs. RHE) in neutral electrolyte, the multivalent Cu-based electrode achieved the nitrate conversion of 99.99 %, NH3 yield rate of 1040.82 µg h−1 cm−2 and NH3 Selectivity of 99.5 %. Furthermore, the electrodes demonstrated high nitrate conversion and good NH3 yield when powered by a small solar photovoltaic panel, suggesting potential for industrial-scale production using renewable energy sources.

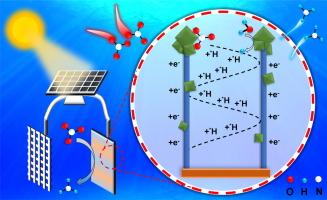
多价多维铜基电极高效电催化硝酸还原制氨的物理和电子结构优化
电化学还原硝酸盐合成氨因其低能耗和环境兼容性而备受关注。为了促进电化学合成氨催化剂的工业化应用,不仅要考虑催化剂的高催化活性和选择性,还要考虑其可扩展的制造工艺和简便的制备方法。本研究采用简单的浸渍还原法设计了一种多价铜基氧化物的多维复合电极。Cu(OH)2 纳米线和 Cu2O 纳米颗粒在泡沫铜上原位生长,形成了多维复合结构。随后,电极通过电化学原位还原转化为 Cu+/Cu0,而微观结构和形态并未发生显著变化。多价 Cu 基氧化物之间的电子相互作用促进了对 NO3- 分子的物理化学吸附,并优化了电子和质子的转移途径。在中性电解质中,当电位为 -0.8 V(相对于 RHE)时,多价 Cu 基电极的硝酸盐转化率达到 99.99%,NH3 产率达到 1040.82 µg h-1 cm-2,NH3 选择性达到 99.5%。此外,在小型太阳能光伏板的驱动下,电极还实现了高硝酸盐转化率和良好的 NH3 产率,这表明电极具有利用可再生能源进行工业规模生产的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


