Preparation of V4+electrolyte by nanofluid-based electrocatalytic reduction of V2O5 for vanadium redox flow batteries
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
The electrolyte employed in the vanadium redox flow battery (VRFB) is typically produced on an industrial scale through a secure and contaminant-free electrolysis process. However, the electrolysis rate is relatively sluggish, leading to elevated energy consumption. Herein, we propose a cost-effective method for electrolytic production of V(IV) electrolytes with carboxyl-functionalized multi-walled carbon nanotubes (MWCNTs-COOH) as the electrocatalyst and explore their effects on the electrolysis process. The results indicate that adding MWCNTs-COOH nanoparticles enhances the reactive sites of the electrode, thus improving the electrochemical activity of the electrolyte. The 0.1 wt% nanoparticle demonstrates the optimal catalytic performance for reducing V(V) to V(IV). Compared to the traditional electrolysis method, the proposed approach exhibits a 6.67 % increase in electrolysis rate, a 15.57 % reduction in energy consumption, and a notable relief in electrode corrosion. Furthermore, cyclic charge/discharge experiments illustrate that the prepared nanofluidic electrolyte exhibits superior performance in terms of voltage efficiency, Coulombic efficiency, energy efficiency, and discharge capacity retention. This invention offers a novel concept for vanadium electrolyte preparation, which reduces energy consumption and cost and improves the system's energy efficiency, showing great promise for practical VRFB applications.

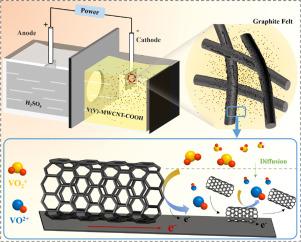
纳米流体电催化还原钒氧化还原液电池用V2O5制备V4+电解液
钒氧化还原液流电池(VRFB)中使用的电解液通常是通过安全、无污染的电解工艺进行工业化生产的。然而,电解速度相对较慢,导致能耗升高。在此,我们提出了一种以羧基功能化多壁碳纳米管(MWCNTs-COOH)为电催化剂的经济高效的 V(IV)电解质电解生产方法,并探讨了其对电解过程的影响。结果表明,添加 MWCNTs-COOH 纳米粒子可增强电极的反应位点,从而提高电解液的电化学活性。0.1 wt% 的纳米粒子在将 V(V)还原为 V(IV)方面表现出最佳催化性能。与传统的电解方法相比,该方法的电解率提高了 6.67%,能耗降低了 15.57%,电极腐蚀明显减轻。此外,循环充放电实验表明,制备的纳米流体电解液在电压效率、库仑效率、能效和放电容量保持率方面都表现出卓越的性能。本发明为钒电解液的制备提供了一种新的概念,既降低了能耗和成本,又提高了系统的能效,在 VRFB 的实际应用中大有可为。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
文献相关原料
公司名称
产品信息
阿拉丁
MWCNT-COOH nanoparticles
阿拉丁
V2O5
阿拉丁
MWCNT-COOH nanoparticles
阿拉丁
V<sub>2</sub>O<sub>5</sub>
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


