Understanding the Unique Selectivity of Cobalt Phthalocyanine in Multielectron Reduction of Carbon Dioxide
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Metal–nitrogen–carbon (M–N–C) single-atom catalysts (SACs) have emerged as promising heterogeneous electrocatalysts for the CO2 reduction reaction (CO2RR). However, the predominant production of CO over multielectron products remains a challenge for most M–N–C SACs, with the exception of cobalt phthalocyanine (CoPc). In this study, the comparison of CoPc and a series of analogous M–N–C SACs was systematically investigated using density functional theory calculations to unravel the factors contributing to the selectivity of CoPc in catalyzing multielectron CO2RR. The relationship between the selectivity and the electronic configuration of M–N–C SACs was revealed. The half-filled dz2 orbital of the cobalt ion lead to moderate chemisorption of *CO on CoPc, enabling the subsequent protonation of *CO. In addition, we identified a unique type of hydrogen bond in which the C atom of *CO acts as the proton acceptor (C···H–O hydrogen bond), which significantly promotes the proton transfer to *CO and selectivity for multielectron products. Only the *CO on CoPc was observed to form the C···H–O hydrogen bond, elucidating the unique multielectron CO2RR performance of CoPc. In addition, we further elucidated the formation mechanism of the C···H–O hydrogen bond, which provides an alternative strategy to accelerate proton transfer in electrochemical reactions by utilizing this unconventional hydrogen bond.
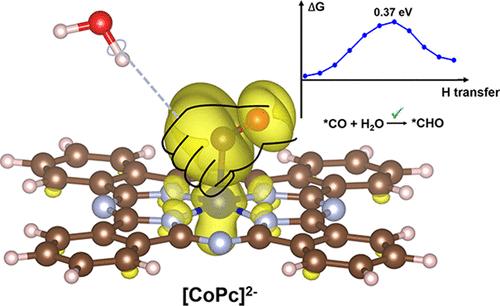
了解酞菁钴在二氧化碳多电子还原中的独特选择性
金属-氮-碳(M-N-C)单原子催化剂(SAC)已成为二氧化碳还原反应(CO2RR)中很有前途的异质电催化剂。然而,除了酞菁钴(CoPc)之外,大多数 M-N-C SACs 的主要生成物仍是二氧化碳,而不是多电子产物。本研究利用密度泛函理论计算对 CoPc 和一系列类似的 M-N-C SAC 进行了系统研究,以揭示 CoPc 在催化多电子 CO2RR 时的选择性因素。研究揭示了选择性与 M-N-C SACs 电子构型之间的关系。钴离子半填充的 dz2 轨道导致 *CO 在 CoPc 上产生适度的化学吸附,从而使 *CO 随后发生质子化反应。此外,我们还发现了一种独特的氢键类型,其中 *CO 的 C 原子充当质子受体(C--H-O 氢键),这极大地促进了质子向 *CO 的转移和多电子产物的选择性。据观察,只有 CoPc 上的 *CO 形成了 C-H-O 氢键,从而阐明了 CoPc 独特的多电子 CO2RR 性能。此外,我们还进一步阐明了 C-H-O 氢键的形成机理,为利用这种非常规氢键加速电化学反应中的质子转移提供了另一种策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


