One-Pot and Gram-Scale Synthesis of Fe-Based Nanozymes with Tunable O2 Activation Pathway and Specificity Between Associated Enzymatic Reactions
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Nanozymes have recently gained attention for their low cost and high stability. However, unlike natural enzymes, they often exhibit multiple enzyme-like activities, complicating their use in selective bioassays. Since H2O2 and O2 are common substrates in these reactions, controlling their activation—and thus reaction specificity—is crucial. Recent advances in tuning the chemical state of cerium have enabled control over H2O2 activation pathways for tunable peroxidase/haloperoxidase-like activities. In contrast, the control of O2 activation on an element in oxidase/laccase nanozymes and the impact of its chemical state on these activities remains unexplored. Herein, a facile one-pot method is presented for the gram-scale synthesis of Fe-based nanozymes with tunable compositions of Fe3O4 and Fe3C by adjusting preparation temperatures. The Fe3O4-containing samples exhibit superior laccase-like activity, while the Fe3C-containing counterparts demonstrate better oxidase-like activity. This divergent O2 activation behavior is linked to their surface Fe species: the abundant reactive Fe2+ in Fe3O4 promotes laccase-like activity via Fe3+-superoxo formation, whereas metallic Fe in Fe3C facilitates OH radical generation for oxidase-like activity. Controlled O2 activation pathways in these Fe-based nanozymes demonstrate improved sensitivity in the corresponding biomolecule detection, which should inform the design of nanozymes with enhanced activity and specificity.
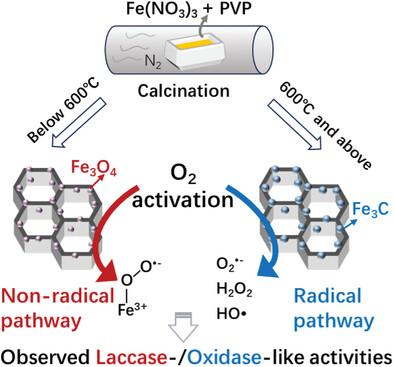
一锅和克级合成具有可调氧激活途径的铁基纳米酶及其相关酶反应的特异性
近年来,纳米酶以其低成本和高稳定性而备受关注。然而,与天然酶不同,它们通常表现出多种酶样活性,使其在选择性生物测定中的应用复杂化。由于H2O2和O2是这些反应中常见的底物,因此控制它们的活化——从而控制反应的特异性——是至关重要的。最近在调整铈的化学状态方面取得的进展,使我们能够控制H2O2激活途径,从而调节过氧化物酶/卤素过氧化物酶样活性。相比之下,氧化酶/漆酶纳米酶中O2活化的控制及其化学状态对这些活性的影响尚不清楚。本文提出了一种简单的一锅法,通过调节制备温度,可调节Fe3O4和Fe3C组成的铁基纳米酶的克级合成。含fe3o4的样品表现出较好的类漆酶活性,而含fe3c的样品表现出较好的类氧化酶活性。这种不同的氧活化行为与它们表面的铁种类有关:Fe3O4中丰富的活性Fe2+通过Fe3+-超氧形成促进了漆酶样活性,而Fe3C中的金属铁促进了OH自由基的生成,从而促进了氧化酶样活性。这些铁基纳米酶的可控氧激活途径在相应的生物分子检测中表现出更高的灵敏度,这应该为设计具有更高活性和特异性的纳米酶提供信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


