Contrasting Effects of Catecholate and Hydroxamate Siderophores on Molybdenite Dissolution
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Molybdenum (Mo) is essential for many enzymes but is often sequestered within minerals, rendering it not readily bioavailable. Metallophores, metabolites secreted by microorganisms and plants, promote mineral dissolution to increase the metal bioavailability. However, interactions between metallophores and Mo-bearing minerals remain unclear. In this study, catecholate protochelin and hydroxamate desferrioxamine B (DFOB) were utilized to examine their effects on dissolution of the common Mo-bearing mineral, molybdenite (MoS2), under both oxic and anoxic conditions. Protochelin promoted molybdenite dissolution under oxic conditions, with the formation of MoO3 on the surface and Mo-siderophore complexes in solution. This was attributed to air-oxidation of both molybdenite and protochelin, as evidenced by lack of dissolution under anoxic conditions but enhanced dissolution by either preoxidized protochelin or preoxidized molybdenite. Liquid chromatography–mass spectroscopy, X-ray photoelectron spectroscopy, and time-of-flight secondary ion mass spectrometry analyses revealed degradation of protochelin and adsorptions of its byproducts on molybdenite surface to promote dissolution. Conversely, DFOB inhibited molybdenite dissolution under both oxic and anoxic conditions, likely attributed to surface adsorption of DFOB and its weak complexation with Mo(VI) at the circumneutral pH. This work highlights the need to consider the balance between promoting and inhibitory effects of different metallophores on Mo-mineral dissolution.
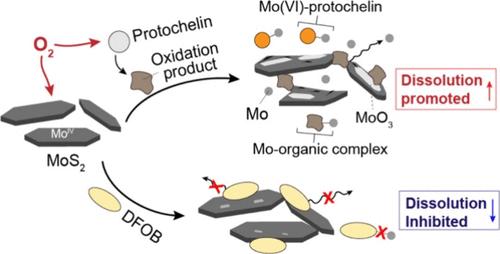
儿茶酸盐和羟氨酸盐苷元对钼矿溶解的不同影响
钼(Mo)是许多酶所必需的元素,但往往被螯合在矿物质中,不易被生物利用。金属团是微生物和植物分泌的代谢物,可促进矿物溶解,提高金属的生物利用率。然而,金属噬菌体与含钼矿物之间的相互作用仍不清楚。在本研究中,利用儿茶酸盐原ochelin 和羟基氨基甲酸酯去铁胺 B(DFOB)研究了它们在缺氧和缺氧条件下对常见含钼矿物--辉钼矿(MoS2)溶解的影响。在缺氧条件下,Protochelin 促进了辉钼矿的溶解,在表面形成了 MoO3,在溶液中形成了钼苷复合体。这归因于辉钼矿和原菱锰矿的空气氧化作用,具体表现为在缺氧条件下不溶解,但预氧化的原菱锰矿或预氧化的辉钼矿会促进溶解。液相色谱-质谱、X 射线光电子能谱和飞行时间二次离子质谱分析表明,原螯合烷发生了降解,其副产品吸附在辉钼矿表面,从而促进了溶解。相反,在缺氧和缺氧条件下,DFOB 都会抑制辉钼矿的溶解,这可能是由于 DFOB 的表面吸附及其在中性 pH 值下与 Mo(VI) 的弱络合作用。这项研究强调了考虑不同金属团对钼矿溶解的促进和抑制作用之间平衡的必要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


