Tin-Chelated Trisphosphineoxide Scorpionate Rare-Earth Porphyrinate Complexes: Synthesis and Photophysical Properties
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
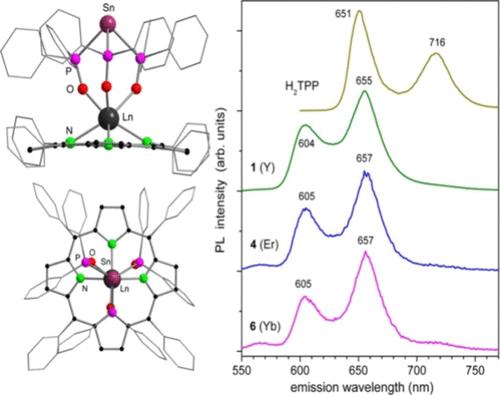
A series of seven-coordinated monoporphyrinate rare-earth(III) complexes featuring a novel tripodal tin-chelated trisphosphineoxide scorpionate ligand with the general formula [(TPP)Ln(PPh2O)3Sn] (Ln = Y, La, Dy, Er, Ho, Yb; TPP = 5,10,15,20-tetraphenylporphyrinate) were synthesized by reactions of the potassium tripodal scorpionate ligand [Sn(PPh2O)3K] with porphyrinate rare-earth metal chlorides [(TPP)LnCl(dme)] (Ln = Y, Dy, Er, Ho, Yb) or porphyrinate lanthanum borohydride [(TPP)LaBH4(thf)2]. The complexes were characterized by single-crystal X-ray diffraction, NMR spectroscopy, and ion mobility mass spectrometry. All complexes emit weak red TPP-based fluorescence, accompanied by near-infrared emission of Er, Ho (rather weak), and Yb (relatively intense with a quantum yield of 1% in dichloromethane solution) of the corresponding complexes. Despite the low intensity, the red fluorescence is characteristic (as referred to the parent free-base TPP) and can be used together with optical absorption for analytical evaluation. Similar photophysical properties can be expected for monoporphyrinate rare-earth metal complexes of other tripodal ligands with a similar binding to the (TPP)Ln moiety.
锡螯合三膦氧化物蝎酸稀土卟啉酸络合物:合成与光物理性质
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


