MR Imaging Reveals Dynamic Aggregation of Multivalent Glycoconjugates in Aqueous Solution
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
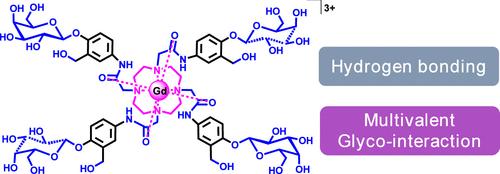
Glycoconjugates forming from the conjugation of carbohydrates to other biomolecules, such as proteins, lipids, or other carbohydrates, are essential components of mammalian cells and are involved in numerous biological processes. Due to the capability of sugars to form multiple hydrogen bonds, many synthetic glycoconjugates are desirable biocompatible platforms for imaging, diagnostics, drugs, and supramolecular self-assemblies. Herein, we present a multimeric galactose functionalized paramagnetic gadolinium (Gd(III)) chelate that displays spontaneous dynamic aggregation in aqueous conditions. The dynamic aggregation of the Gd(III) complex was shown by the concentration-dependent magnetic resonance (MR) relaxation measurements, nuclear magnetic resonance dispersion (NMRD) analysis, and dynamic light scattering (DLS). Notably, these data showed a nonlinear relationship between magnetic resonance relaxation rate and concentrations (0.03–1.35 mM), and a large DLS hydrodynamic radius was observed in the high-concentration solutions. MR phantom images were acquired to visualize real-time dynamic aggregation behaviors in aqueous solutions. The in situ visualization of the dynamic self-assembling process of multivalent glycoconjugates has rarely been reported.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


