Electro-upcycling polyethylene glycol aqueous solutions in a proton exchange membrane electrolyser
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
This study addresses the electro-upcycling, at low temperature and neutral pH conditions, of aqueous solutions of polyethylene glycol (PEG). For this purpose, a Proton-Exchange Membrane (PEM) reactor with Pt/C-based electrodes has been used. The electrooxidation performances of ethylene glycol (EG) and PEG were evaluated for various concentrations in aqueous solutions, temperatures and PEG molecular weights (MW). PEG was successfully electro-oxidized from 0.4 V, reaching current densities above 100 mA cm-2 at 0.8 V and 80 °C using oligoethylene glycols up to 400 g mol-1. Size-exclusion chromatography and NMR analysis evidenced the possibility to cleave –C–O–C– bonds of heavy PEG (1000, 4000 g mol-1), proving the possibility of depolymerizing PEG and recovering the monomer at low cell voltages and low temperatures. The production and purity of hydrogen in the cathode compartment of the electrolyser was confirmed by mass spectrometry. These results may open new perspectives in the development of electro-upcycling of PEG in water effluents.
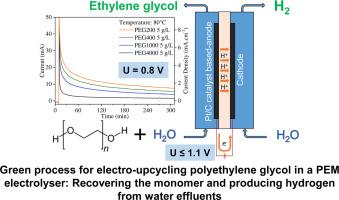
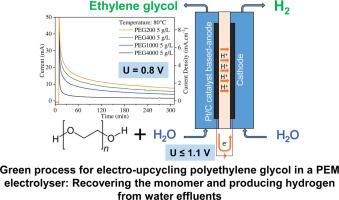
质子交换膜电解槽中聚乙二醇水溶液的电循环利用
本研究解决了低温和中性pH条件下聚乙二醇(PEG)水溶液的电升级回收。为此,使用了质子交换膜(PEM)反应器和铂/碳基电极。考察了乙二醇(EG)和聚乙二醇(PEG)在不同浓度、不同温度、不同分子量水溶液中的电氧化性能。在0.8 V和80°C条件下,PEG成功地在0.4 V下电氧化,达到超过100 mA cm-2的电流密度,使用的聚乙二醇高达400 g mol-1。尺寸排除层析和核磁共振分析证明了重质PEG(1000、4000 g mol-1)的- c - o - c -键的可能性,证明了在低电池电压和低温下解聚PEG和回收单体的可能性。用质谱法测定了电解槽阴极室氢气的产量和纯度。这些结果可能为污水中聚乙二醇的电升级利用的发展开辟新的前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


