Incorporation of crown ether into PEI-polyamide nanofiltration membrane for efficient Mg2+/Li+ separation
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Positive charged nanofiltration (NF) membrane have been triggered for Li+ extraction from brine driven by the Donnan effect. However, achieving selective Li+ transport channels remains a significant challenge, particularly in brines with a high Mg2+/Li+ mass ratio. In this work, a rigid crown ether was incorporated into the polyethyleneimine (PEI) based polyamide (PA) membrane for enhancing the robust selectivity of the typical positive charged membrane. Di(aminobenzo)-18-crown-6 (DAB18C6) was chosen as an additional aqueous phase monomer alongside PEI, with both reacting with trimesoyl chloride (TMC) to fabricate membranes via interfacial polymerization (IP). Due to the rigidity and the unique selectivity for alkali metal ions, DAB18C6 molecules not only expand the interval of the tightly stacking PEI-TMC chain in the PA layer, which resulting in high permeability, but also provide selective channels for Li+ transport. Therefore, the obtained DAB18C6@PEI@PSF membrane achieving the simultaneous enhancement in permeability (8.2 L·m−2·h−1·bar−1) and ion separation factor (SLi,Mg = 23). Molecular dynamics demonstrated that the DAB18C6 exerts a stronger force on Li+ compared to Mg2+, which facilitates the transmembrane transport of the monovalent ion and achieves a high separation factor. After three-stage NF treatment process for a simulated salt lake brine with initial Mg2+/Li+ ratio of 61.5, a permeability liquid with low Mg2+/Li+ ratio of only 0.16 was obtained, indicating that the DAB18C6@PEI@PSF membrane has promising potential application in the field of high mass ratio Mg2+/Li+ separation.
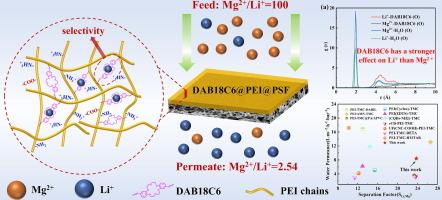

冠醚掺入pei -聚酰胺纳滤膜用于Mg2+/Li+的高效分离
在唐南效应的驱动下,带正电荷的纳滤膜已被用于从盐水中提取 Li+。然而,实现选择性 Li+ 传输通道仍是一项重大挑战,尤其是在 Mg2+/Li+ 质量比很高的盐水中。在这项工作中,在基于聚乙烯亚胺(PEI)的聚酰胺(PA)膜中加入了一种刚性冠醚,以增强典型正电荷膜的稳健选择性。二(氨基苯并)-18-冠醚-6(DAB18C6)与 PEI 一起被选为额外的水相单体,二者与三甲基甲酰氯(TMC)反应,通过界面聚合(IP)制造膜。由于 DAB18C6 分子的刚性和对碱金属离子的独特选择性,它不仅扩大了 PA 层中紧密堆叠的 PEI-TMC 链的间隔,从而实现了高渗透性,而且还为 Li+ 的传输提供了选择性通道。因此,所获得的 DAB18C6@PEI@PSF 膜实现了渗透率(8.2 L-m-2-h-1-bar-1)和离子分离因子(SLi,Mg = 23)的同时提高。分子动力学证明,与 Mg2+ 相比,DAB18C6 对 Li+ 的作用力更大,这有利于单价离子的跨膜传输,并实现了高分离因子。在对初始 Mg2+/Li+ 比率为 61.5 的模拟盐湖卤水进行三级 NF 处理后,得到了 Mg2+/Li+ 比率仅为 0.16 的低渗透液,这表明 DAB18C6@PEI@PSF 膜在高质比 Mg2+/Li+ 分离领域具有广阔的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


