Mechanistic insights into RuⅣ/RuⅢ and ·OH-co-participated selective oxidation of thioethers into sulfoxides and sulfones over a Ru-Co3O4 electrocatalyst
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
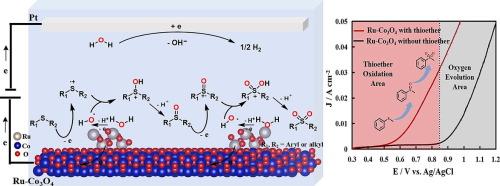
Heterogeneous electrocatalysis is an advanced tactics to oxidize organic sulfides into value-added products, concurrently being accompanied with hydrogen production in the aqueous electrolyte. However, the insufficient oxidizing ability of redox mediators and competitive oxygen evolution reaction (OER) inhibited the late-stage oxygenation of thioethers to produce sulfones. Herein, a RuO2-loaded Co3O4 electrocatalyst (Ru-Co3O4) was constructed to oxidize thioethers at the potential range of 0.7–0.8 V (vs. Ag/AgCl) at which the OER did not sharply occur, and various sulfoxides and sulfones were produced selectively with moderate to good yields. Mechanism studies revealed that the Ru-Co3O4 electrocatalyst afforded Ru4+ active species and hydroxyl radicals (·OH) at a low potential. The generated RuⅣ/RuⅢ redox couple was responsible for the oxidation of sulfides into sulfur-related cation radicals which then reacted with ·OH and deprotonated to form oxygenation products. This work provided a reasonable proposal for the design of heterogeneous electrocatalysts, which could effectively drive the organic oxygenation reactions.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


