Enhanced interfacial bonding strategy via the molecular encapsulation effect for durable superhydrophobic coatings
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
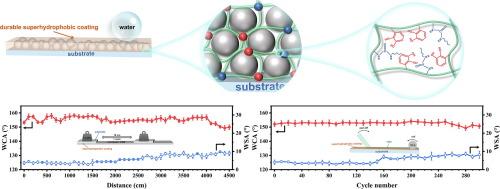
Superhydrophobic coatings have attracted widespread attention for their high versatility and scalability in various water-involved application scenarios. Developing superhydrophobic coatings with excellent durability is important, yet extremely challenging. Since the interfacial bonding offered by the essential low-surface-energy property is too limited to maintain surface textures. Herein, a well-tailored strategy is proposed to address the interfacial weakness by introducing efficient adhesive functional moieties into the low surface energy polymer structures employed for constructing superhydrophobic coatings. Catechol and urea groups were selected as key functional moieties and were homogeneously dispersed within the silicone network by being constructed as crosslinkers. In addition, the urea moiety effectively suppressed the oxidation failure of the catechol moiety because of the molecular encapsulation effect which functioned through the hydrogen-bonded intermolecular interactions. Owing to the synergistic enhancement of the two adhesion functional moieties, the optimized superhydrophobic coating showed over fivefold boost in mechanical stability, tolerating 40 m of sandpaper abrasion and 250 cycles of tape peeling. Superhydrophobicity was also well kept after various harsh environmental tests including chemical corrosion, UV aging, and extreme temperatures. This novel approach indicates a feasible way to enhance durability and will contribute to the practical application of superhydrophobic coatings.
通过分子包封效应增强界面键合策略的耐久超疏水涂层
超疏水涂层因其在各种涉水应用场景中的高度通用性和可扩展性而受到广泛关注。开发具有出色耐久性的超疏水涂层非常重要,但也极具挑战性。因为低表面能特性所提供的界面结合力非常有限,无法保持表面纹理。在此,我们提出了一种量身定制的策略,通过在用于构建超疏水涂层的低表面能聚合物结构中引入高效粘合功能分子来解决界面薄弱问题。我们选择了儿茶酚和脲基作为关键的功能分子,并通过构建交联剂使其均匀地分散在有机硅网络中。此外,脲基团通过氢键分子间相互作用产生的分子封装效应,有效抑制了儿茶酚基团的氧化失效。由于两种粘附功能分子的协同增效作用,优化后的超疏水涂层的机械稳定性提高了五倍以上,可以承受 40 米的砂纸磨损和 250 次的胶带剥离。在经过各种严酷的环境测试(包括化学腐蚀、紫外线老化和极端温度)后,超疏水性能也得到了很好的保持。这种新方法为提高耐久性提供了一种可行的途径,将有助于超疏水涂层的实际应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
文献相关原料
公司名称
产品信息
阿拉丁
Bisphenol A (BPA)
阿拉丁
isophorone diisocyanate (IPDI)
阿拉丁
(3-aminopropyl) triethoxysilane (APTES)
阿拉丁
dibutyltin dilaurate (DBTDL)
阿拉丁
lithium perchlorate (LiClO4)
阿拉丁
Bisphenol A
阿拉丁
Isophorone diisocyanate
阿拉丁
(3-aminopropyl) triethoxysilane
阿拉丁
Dibutyltin dilaurate
阿拉丁
2-iodoxybenzoic acid
阿拉丁
Lithium perchlorate
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


