Phase Engineering of Zirconia Support Promotes the Catalytic Dehydrogenation of Formic Acid by Pd Active Sites
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
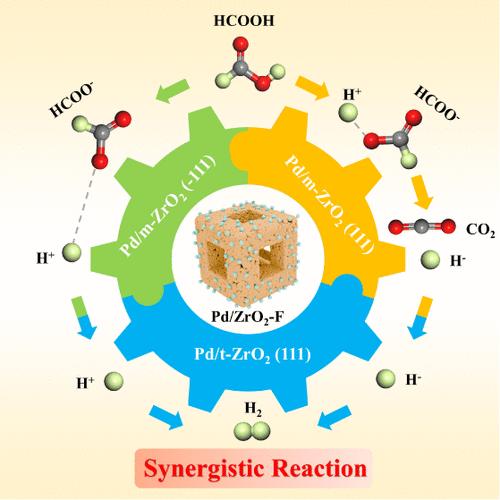
The development of Pd-based catalysts with outstanding activity and stability can further promote the hydrogen storage application of formic acid (FA). Regulating the support structure is an effective strategy for enhancing active sites in heterogeneous catalytic systems. This study prepared three types of nanosized ZrO2 through phase engineering to support Pd metal and investigated the implications of support structure on the microenvironment of active sites, thus revealing the structure–activity relationship of the catalysts. The hollow nanoframes like Pd/ZrO2-F with a moderate t-ZrO2 content exhibit remarkable stability and catalytic performance with a TOF of 1348 h–1 at an ambient temperature. Density functional theory (DFT) calculations verify that the crystal phase of ZrO2 can dramatically affect the metal–support interaction and change the Pd electronic state. Moreover, the dehydrogenation energy profiles reveal the synergy effect between ZrO2 phases on Pd active sites in the reaction. Pd/m-ZrO2 is more conducive to the dissociation of FA, and Pd/t-ZrO2 has energy advantages in hydrogen recombination. This work provides a new perspective for understanding the synergistic effect of the zirconia crystal phase on formic acid dehydrogenation by Pd active sites.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


