Tunable Optical Properties, Enhanced Photoluminescence, and Thermostability of CaO: Eu2+/3+ Phosphors by Codoping Cl– Ions
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Europium-activated calcium oxide (CaO: Eu) phosphor shows great potential in the fabrication of light-emitting diodes (LEDs). However, its luminescent intensity and thermostability still need to be further enhanced. In this work, the photoluminescence property and thermostability of CaO: Eu2+/3+ were improved significantly by codoping a few of Cl– ions into it. Compared with CaO: Eu2+/3+ samples, CaO: Eu2+/3+, Cl– phosphors not only have tunable luminescence but also show stronger luminescence intensity and thermal stability. The emission color of phosphors can be tuned by changing the excitation wavelength; typically, strong red/blue light can be displayed under 250/362 nm excitation. More importantly, both the red and blue emissions will increase simultaneously with increasing codoped Cl– content. When the Cl– ion content is 0.06, the emission intensity of CaO: 0.01Eu2+/3+, 0.06Cl– reaches its maximum and its red and blue emission intensities increase by approximate 2.5 times and 13.2 times, respectively. The results show that CaO: Eu2+/3+, Cl– phosphors have potential application value in LEDs and anti-counterfeiting.
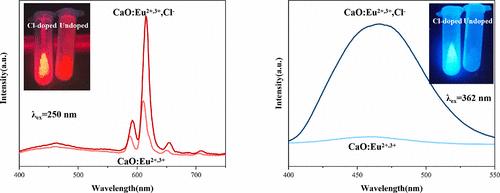
通过共掺杂Cl -离子,CaO: Eu2+/3+荧光粉的可调光学性质、增强光致发光和热稳定性
铕活化氧化钙(CaO: Eu)荧光粉在发光二极管(led)的制造中显示出巨大的潜力。但其发光强度和热稳定性仍需进一步提高。在本研究中,通过在CaO: Eu2+/3+中共掺杂少量Cl -离子,显著提高了CaO: Eu2+/3+的光致发光性能和热稳定性。与CaO: Eu2+/3+样品相比,CaO: Eu2+/3+, Cl -荧光粉不仅发光可调,而且具有更强的发光强度和热稳定性。通过改变激发波长可以调节荧光粉的发射颜色;通常,在250/362 nm的激发下可以显示出强烈的红/蓝光。更重要的是,随着共掺杂Cl -含量的增加,红色和蓝色的发射量同时增加。当Cl -离子含量为0.06时,CaO: 0.01Eu2+/3+, 0.06Cl -的发射强度达到最大值,其红色和蓝色发射强度分别增加了约2.5倍和13.2倍。结果表明,CaO: Eu2+/3+, Cl -荧光粉在led和防伪方面具有潜在的应用价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


