Mitochondria Localized Anticancer Iridium(III) Prodrugs for Targeted Delivery of Myeloid Cell Leukemia-1 (Mcl-1) Inhibitors and Cytotoxic Iridium(III) Complex
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
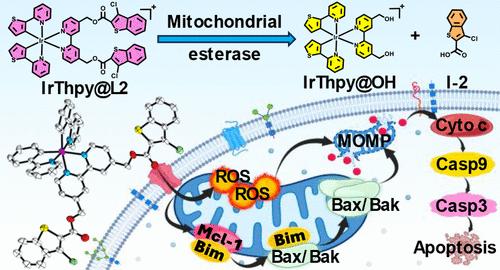
Myeloid cell leukemia-1 (Mcl-1) is an antiapoptotic oncoprotein overexpressed in several malignancies and acts as one of the promising therapeutic targets for cancer. Even though there are several small molecule based Mcl-1 inhibitors reported, the delivery of Mcl-1 inhibitor at the target site is quite challenging. In this regard, we developed a series of mitochondria targeting luminescent cyclometalated iridium(III) prodrugs bearing Mcl-1 inhibitors via ester linkage due to the presence of Mcl-1 protein in the outer mitochondrial membrane. Among the synthesized prodrugs, IrThpy@L2 was found to exhibit the potent cytotoxicity (IC50 = 30.93 nM) against HCT116 cell line when compared with bare Mcl-1 inhibitors (IC50 > 100 μM). Mechanistic studies further revealed that IrThpy@L2 quickly gets internalized inside the mitochondria of HCT116 cells and undergoes activation in the presence of overexpressed esterase which leads to the release of two cytotoxic species i.e. Mcl-1 inhibitors (I-2) and cytotoxic iridium(III) complex (IrThpy@OH). The improved cytotoxicity of IrThpy@L2 is due to the mitochondria targeting ability of iridium(III) prodrug, subsequent esterase activated release of I-2 to inhibit Mcl-1 protein and IrThpy@OH to generate reactive oxygen species (ROS). After prodrug activation, the released cytotoxic species cause mitochondrial membrane depolarization, activate a cascade of mitochondria-mediated cell death events, and arrest the cell cycle in S-phase which leads to apoptosis. The potent anticancer activity of IrThpy@L2 was further evident from the drastic morphological changes, size reduction in the solid tumor mimicking 3D multicellular tumor spheroids (MCTS) of HCT116.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


