Ligands Induced the Growth of Colloidal AgInS2 Nanoparticles with Tuned Structure and Photoluminescence Property
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
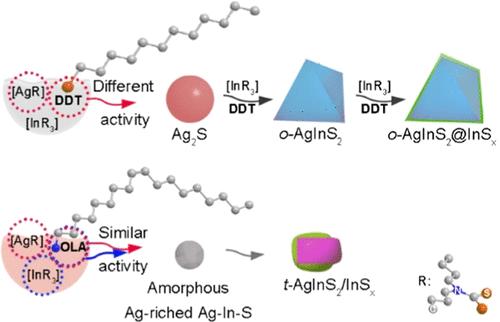
Understanding the growth mechanism of AgInS2 nanoparticles could benefit the designed growth of I–III–VI2 nanomaterials and their applications in photonics, optoelectronics, etc. Herein, using tris(dibutyldithiocarbamate) indium(III) [In((C4H9)2NCS2)3] ([InR3]) and dibutyldithiocarbamate silver(I) [Ag((C4H9)2NCS2)] ([AgR]) precursors, AgInS2-based nanoparticles with different structures have been synthesized in a controlled manner through a one-pot approach via different growth mechanisms in 1-dodecanethiol (DDT) and oleylamine (OLA), respectively. The DDT and OLA could participate in the decomposition of precursors; thus, the [AgR]/DDT, [InR3]/DDT, [AgR]/OLA, and [InR3]/OLA were used herein to describe the decomposition steps. In DDT, the decomposition activity of [AgR]/DDT was much higher than that of [InR3]/DDT; thus, the sequential decomposition of [AgR]/DDT and [InR3]/DDT led to the formation of the Ag2S nanoparticles intermediate first, which then reacted with [InR3]/DDT to form metastable o-AgInS2 nanoparticles via the cation exchange and alloy process, and finally evolved into o-AgInS2@InSx core@shell nanoparticles, while in OLA, the decomposition activity of [AgR]/OLA was slightly higher than that of [InR3]/OLA. Thus, the quasi-co-decomposition of [AgR]/OLA and [InR3]/OLA led to the formation of Ag-rich Ag–In–S amorphous nanoparticles intermediate first and then quickly evolved into stable t-AgInS2/InSx nanoparticles. In addition, the photoluminescence quantum yield (PLQY) of t-AgInS2/InSx nanoparticles was higher than that of o-AgInS2@InSx nanoparticles.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


