Quantity effect of heteroatom incorporation on the oxygen evolution mechanism in ruthenium oxide
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Research on ruthenium oxide (RuO2) catalysts as alternatives to Ir-based catalysts for the acidic oxygen evolution reaction (OER) has focused on enhancing activity and stability by incorporating heteroatoms. However, the relationship between the amount of incorporated heteroatom and the OER mechanism remains unclear. Herein, we synthesized rutile manganese-ruthenium solid-solution oxides (MnxRu1-xO2) with varying Mn/Ru ratios to identify factors affecting activity and stability with Mn content. Both experimental and computational results show that increasing Mn content raises the oxidation state of Ru and shifts the OER mechanism from the adsorbate evolution mechanism (AEM) to the lattice oxygen mechanism (LOM). Increased Mn concentration enhances Ru–O bond covalency, leading to lattice oxygen involvement in the OER. The Mn0.2Ru0.8O2 catalyst, with an optimal Mn/Ru ratio, operated stably in a proton exchange membrane water electrolyzer (PEMWE) for 100 h and achieved 3.15 A cm−2 at 1.8 Vcell, surpassing the 2026 Department of Energy activity goal.
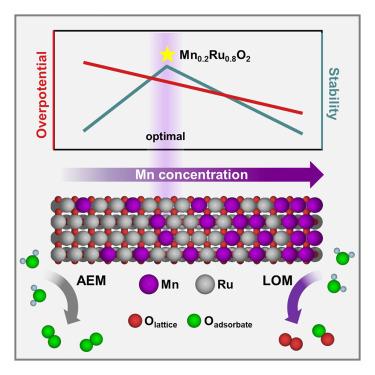

杂原子掺入量对氧化钌析氧机制的影响
氧化钌(RuO2)催化剂作为ir基催化剂在酸性出氧反应(OER)中的替代品的研究主要集中在通过加入杂原子来提高活性和稳定性。然而,杂原子掺入量与OER机制之间的关系尚不清楚。在此,我们合成了不同Mn/Ru比的金红石锰钌固溶体氧化物(MnxRu1-xO2),以确定Mn含量对活性和稳定性的影响因素。实验和计算结果均表明,Mn含量的增加提高了Ru的氧化态,使OER机制从吸附质演化机制(AEM)转变为晶格氧机制(LOM)。Mn浓度的增加提高了Ru-O键的共价,导致OER中晶格氧的参与。Mn0.2Ru0.8O2催化剂具有最佳的Mn/Ru比,在质子交换膜水电解槽(PEMWE)中稳定运行100 h,并在1.8 v电池下达到3.15 a cm - 2,超过了美国能源部2026年的活动目标。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


