Switchable diversification of quaternary ammonium salts using photocatalysis
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Over the past few decades, significant advances have been made in the radical chemistry of amine molecules. However, the radical reactions of related quaternary ammonium salts remain comparatively underexplored. If radicals could be generated from ammonium salts in a controlled manner, this could lead to a method for producing distonic radical cations, offering valuable synthetic applications for quaternary ammonium salts and their tertiary amine derivatives. In this study, we developed a photoredox-catalyzed method for derivatizing quaternary ammonium salts by reacting α-haloalkylammonium salts with olefins. The key to success is the photocatalytic generation of distonic α-ammonio radicals under both oxidative and reductive quenching conditions. This chemistry enables selective and switchable alkylations and alkenylations, affording structurally new quaternary ammonium salts. The utility of this procedure is showcased in the divergent synthesis and derivatization of bioactive quaternary ammonium salts, a deuterated tertiary amine, and the identification of salinity-tolerance-conferring molecules.
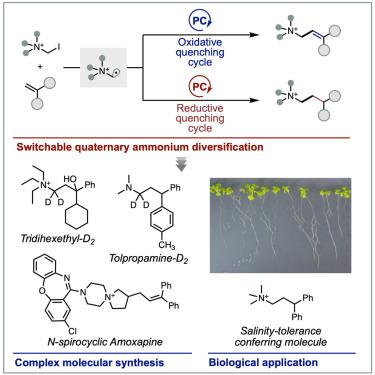

利用光催化实现季铵盐的可切换多样化
在过去的几十年里,胺分子的自由基化学研究取得了重大进展。然而,相关季铵盐的自由基反应研究相对较少。如果能以可控的方式从铵盐中生成自由基,这可能会导致一种生产双离子自由基阳离子的方法,为季铵盐及其叔胺衍生物的合成提供有价值的应用。本研究建立了α-卤代烷基铵盐与烯烃反应衍生季铵盐的光氧化催化方法。成功的关键是在氧化和还原猝灭条件下光催化生成双离子α-氨自由基。这种化学反应使选择性和可切换的烷基化和烯化,提供结构上新的季铵盐。这一过程的实用性在生物活性季铵盐、氘化叔胺的发散合成和衍生化以及耐盐分子的鉴定中得到了体现。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


