Green Hydrogen Production by Chemical Looping Reforming of Wheat Straw Pyrolysis Volatiles via LaNixFe1–xO3@SBA-15
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Using zero-carbon resource biomass with wide sources and abundant reserves as raw materials is of great significance for the sustainable development of energy and the environment to produce hydrogen with green and low carbon. In this study, a LaNixFe1–xO3@SBA-15 perovskite oxygen carrier with strong oxygen storage capacity and controllable lattice transport was prepared, which was used in the chemical looping reforming process of total components of wheat straw pyrolysis volatiles, and it is expected to realize the efficient production of green hydrogen. With volatile conversion, syngas selectivity, and H2/CO as responses, orthogonal experiments were designed and carried out based on the multifactor response surface analysis method to analyze the interaction between factors. The results showed that Ni doping reduced the metal–oxygen bond’s binding energy and improved lattice oxygen’s mobility. LaNi0.8Fe0.2O3@S was considered the best oxygen carrier, whose volatile conversion and syngas selectivity were 98.0 and 84.5% and the output of H2 and syngas was 544.9 and 880.2 mL·g–1, respectively. Multifactor response surface analysis showed that temperature was the key factor of volatile chemical looping reforming, and there was a strong interaction between the amount of oxygen carriers and space velocity. The optimum reaction conditions were 765 °C, 150 mL·min–1·g–1, and 0.82 g of oxygen carriers. The experimental verification showed that the volatile conversion rate was 98.0%, and the predicted value was 98.7%, and the predicted value was basically consistent with the experimental value. Ten cycles showed that LaNi0.8Fe0.2O3@S had excellent reforming performance, the volatile conversion rate had good stability with a maximum fluctuation of less than 2%, the selectivity of syngas remained around 84%, and the CH4 content remained within 2%. This study provided a certain experimental basis for the preparation of green hydrogen by chemical looping reforming of biomass pyrolysis volatiles.
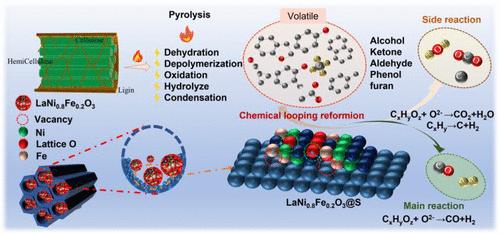
LaNixFe1 - xO3@SBA-15小麦秸秆热解挥发物化学环重整绿色制氢
利用来源广泛、储量丰富的零碳资源生物质为原料,生产绿色低碳制氢对能源和环境的可持续发展具有重要意义。本研究制备了一种储氧能力强、晶格输运可控的LaNixFe1 - xO3@SBA-15钙钛矿氧载体,用于麦秸热解挥发物总组分的化学环重整过程,有望实现绿色氢的高效生产。以挥发性转化率、合成气选择性和H2/CO为响应,基于多因素响应面分析法设计并进行正交试验,分析各因素之间的交互作用。结果表明,Ni的掺杂降低了金属-氧键的结合能,提高了晶格氧的迁移率。LaNi0.8Fe0.2O3@S为最佳氧载体,其挥发性转化率和合成气选择性分别为98.0和84.5%,H2和合成气产量分别为544.9和880.2 mL·g-1。多因素响应面分析表明,温度是挥发性化学环重整的关键因素,载氧量与空速之间存在较强的交互作用。最佳反应条件为765℃,150 mL·min-1·g - 1,载氧量0.82 g。实验验证表明,挥发物转化率为98.0%,预测值为98.7%,预测值与实验值基本一致。10次循环表明,LaNi0.8Fe0.2O3@S重整性能优良,挥发性转化率稳定性好,最大波动小于2%,合成气选择性保持在84%左右,CH4含量保持在2%以内。本研究为生物质热解挥发物化学环重整制备绿色氢提供了一定的实验依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


