Investigation on the Mechanism of Efficient Removal of Phosphorus and Fluorine Impurities from Phosphogypsum by an In Situ Recrystallization Purification Method
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
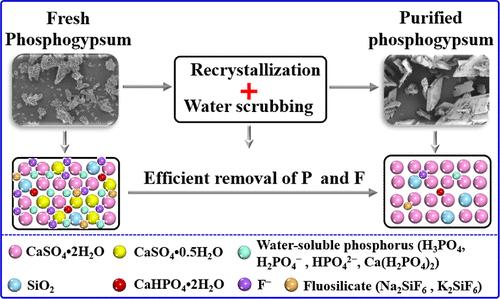
Phosphogypsum, a solid waste produced in the wet phosphoric acid process, can lead to severe environmental pollution. Recently, the resource utilization of phosphogypsum as a raw material for cement retarders and other products has received widespread attention, but it is limited by the high cost of purifying impurities, such as phosphorus and fluorine, contained in phosphogypsum. Herein, a novel method for purifying phosphorus and fluorine in phosphogypsum by in situ recrystallization was developed and has been employed in industrial application successfully. Further, the purification process parameters and mechanism were investigated based on a series of characterizations. The experimental results indicate that the removal efficiencies of phosphorus and fluorine achieve 86.4% and 95.71% under the optimized conditions, respectively. Significantly, during the recrystallization and washing process of fresh phosphogypsum, CaSO4·0.5H2O dissolves and then transforms into larger rhombic CaSO4·2H2O particles. Meanwhile, the impurities, such as water-soluble phosphorus ([H3PO4, H2PO4–, HPO42–, and Ca(H2PO4)2), eutectic phosphorus (CaHPO4·2H2O), and water-soluble fluorine (F–, Na2SiF6, and K2SiF6), carried in fresh phosphogypsum are transferred from the solid phase to the liquid phase, thereby achieving purification of phosphogypsum. This research offers robust theoretical backing and practical guidance for the effective purification of phosphogypsum.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


