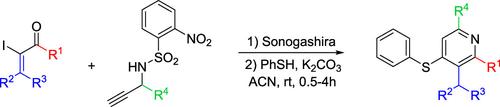
Two-Step Formation of Substituted Pyridines from Iodoenones
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A new access to substituted pyridines was developed from iodoenones. This two-step procedure involves a Sonogashira coupling with a free alkyne containing a nosylamide followed by a thiophenol treatment in basic conditions that triggers nosyl deprotection, a Michael–retro-Michael process, condensation, and isomerization in cascade to yield the heterocycle. This method enables the introduction of different substituents at several pyridine positions. This approach offers new synthetic opportunities to produce heterocycles present in many bioactive compounds.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


