Iodine-Promoted C–H Bond Amination Reaction for the Synthesis of Fused Tricyclic Heteroarenes
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
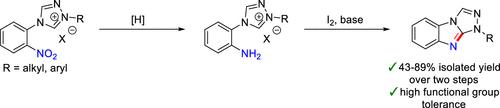
Fused heterocyclic scaffolds, such as benzimidazoles or larger ring systems containing a benzimidazole fragment, are frequently encountered in pharmaceutical compounds and other biologically active molecules. While there are many examples of N9- and/or C3-substituted 9H-benzo[4,5]imidazo[2,1-c][1,2,4]triazoles, current examples of the regioselective preparation of N1-substituted 1H-benzo[4,5]imidazo[2,1-c][1,2,4]triazoles are limited to N1-aryl substituted compounds, which also contain a C3-substituent. Here, we report an iodine-promoted C–H bond amination reaction that allows the selective preparation of 1H-benzo[4,5]imidazo[2,1-c][1,2,4]triazoles with a variety of aryl and alkyl N1-substituents. Not only do these cyclization reactions allow access to a new substitution pattern on the benzo[4,5]imidazo[2,1-c][1,2,4]triazole scaffold, but they are also tolerant toward a wide range of functional groups, including esters, amides, alcohols, alkynes, and alkenes. Our findings expand the synthetic toolbox for the preparation of nitrogen containing fused heteroarenes.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


