CD34+CLDN5+ tumor associated senescent endothelial cells through IGF2-IGF2R signaling increased cholangiocellular phenotype in hepatocellular carcinoma
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
The heterogeneity of hepatocellular carcinoma (HCC) is linked to tumor malignancy and poor prognosis. Nevertheless, the precise mechanisms underlying the development of the cholangiocellular phenotype (CCA) within HCC remain unclear. Emerging studies support that the cross-talk among the host cells within tumor microenvironment (TME) sustains the cancer cell plasticity.
Objectives
This study sought to identify the specific cell types involved in the formation of CCA and to elucidate their functional roles in the progression of HCC.
Methods
Single-cell RNA sequencing was employed to identify the specific cell types involved in the formation of CCA. Both in vitro and vivo analyses were used to identify the tumor-associated senescent ECs and investigate the function in TME. The diethylnitrosamine-induced model was utilized to investigate the interaction between senescent ECs and MSCs, aiming to elucidate their synergistic contributions to the progression of CCA.
Results
Using single-cell RNA sequencing, we identified a distinct senescent-associated subset of endothelial cells (ECs), namely CD34+CLDN5+ ECs, which mainly enriched in tumor tissue. Further, the senescent ECs were observed to secrete IGF2, which recruited mesenchymal stem cells (MSCs) into the TME through IGF2R/MAPK signaling. In primary liver cancer model, MSCs exhibited a strong tumor-promoting effect, increasing the CCA and tumor malignancy after HCC formation. Interestingly, knockdown of IGF2R expression in MSCs inhibited the increase of CCA caused by MSCs in HCC. Meanwhile, it was revealed that MSCs released multiple inflammatory and trophic-related cytokines to enhance the cancer stem cell-like characteristics in HCC cells. Finally, we demonstrated that CEBPβ up-regulated IGF2 expression in tumor senescent ECs by combining with Igf2-promtor-sequence.
Conclusions
Together, our findings illustrated that tumor associated senescent ECs in HCC recruited the MSCs into TME, enhancing cancer stem cell (CSC)-like features of HCC cells and contributing to the CCA formation.
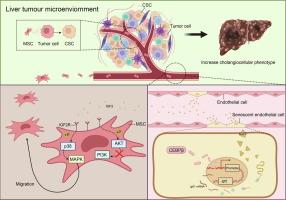
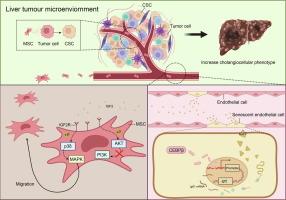
CD34+CLDN5+肿瘤相关衰老内皮细胞通过IGF2-IGF2R信号增加肝细胞癌的胆管细胞表型
肝细胞癌(HCC)的异质性与肿瘤恶性和预后不良有关。然而,HCC中胆管细胞表型(CCA)发生的确切机制尚不清楚。新研究支持肿瘤微环境(tumor microenvironment, TME)内宿主细胞间的串扰维持了癌细胞的可塑性。目的:本研究旨在确定参与CCA形成的特定细胞类型,并阐明它们在HCC进展中的功能作用。方法采用单细胞RNA测序技术鉴定参与CCA形成的特定细胞类型。体外和体内分析鉴定肿瘤相关的衰老ECs,并研究其在TME中的功能。采用二乙基亚硝胺诱导模型研究衰老ec和MSCs之间的相互作用,旨在阐明它们在CCA进展中的协同作用。结果通过单细胞RNA测序,我们发现了内皮细胞(ECs)的一个明显的衰老相关亚群,即CD34+CLDN5+ ECs,主要富集于肿瘤组织中。此外,观察到衰老的ECs分泌IGF2, IGF2通过IGF2R/MAPK信号将间充质干细胞(MSCs)募集到TME中。在原发性肝癌模型中,MSCs表现出较强的促瘤作用,增加HCC形成后的CCA和肿瘤恶性程度。有趣的是,MSCs中IGF2R表达的下调抑制了HCC中MSCs引起的CCA的增加。同时,MSCs释放多种炎症和营养相关细胞因子,增强HCC细胞的癌干细胞样特征。最后,我们证明CEBPβ通过与IGF2启动子序列结合,上调肿瘤衰老ec中IGF2的表达。总之,我们的研究结果表明,HCC中与肿瘤相关的衰老ECs将MSCs招募到TME中,增强HCC细胞的癌干细胞(CSC)样特征,并促进CCA的形成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


