P-doped bimetallic sulfide to boost water dissociation and moderate H* adsorption for efficient alkaline hydrogen evolution
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The development of electrocatalysts for hydrogen evolution reaction (HER) with high catalytic activity and stability in alkaline medium remains a significant challenge. Herein, a catalyst (namely Zn0.05Ni3S2-P) was designed to achieve the excellent alkaline HER performance owing to the boosted water dissociation and moderate H* adsorption energy. At the current density of 10 mA cm−2, the overpotential of Zn0.05Ni3S2-P is 143 mV. In addition, the Tafel slope and the charge transfer resistance of Zn0.05Ni3S2-P are reduced, and the electrochemical active surface area (ECSA) is expanded. Density functional theory (DFT) calculations show that the appropriate proportion of Ni to Zn optimizes the H* adsorption energy of the catalyst. The introduction of P cooperates with S to activate the water dissociation, reduce the H2O dissociation energy barrier, and accelerate the reaction kinetics. Moreover, in the process of electrolytic water splitting at 100 mA cm−2 current density, the voltage is stable at 2.4 V, the hydrogen production rate is maintained at 1.84 mmol h-1, and the Faraday efficiency of hydrogen production is nearly 100 %. This work simultaneously considers the importance of water dissociation and H* adsorption energy in alkaline HER, and provides a strategy for the design of highly active alkaline HER catalysts.

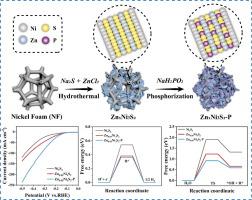
p掺杂双金属硫化物促进水解离和适度的H*吸附有效的碱性析氢
开发在碱性介质中具有高催化活性和稳定性的氢进化反应(HER)电催化剂仍是一项重大挑战。在此,我们设计了一种催化剂(即 Zn0.05Ni3S2-P),该催化剂具有促进水解离和适度的 H* 吸附能,因而在碱性介质中具有优异的氢进化反应性能。在电流密度为 10mA cm-2 时,Zn0.05Ni3S2-P 的过电位为 143 mV。此外,Zn0.05Ni3S2-P 的塔菲尔斜率和电荷转移电阻降低,电化学活性表面积(ECSA)扩大。密度泛函理论(DFT)计算表明,适当比例的 Ni 与 Zn 可优化催化剂的 H* 吸附能。P 的引入与 S 相互配合,激活了水的解离,降低了 H2O 的解离能垒,加速了反应动力学。此外,在电流密度为 100 mA cm-2 的电解水分裂过程中,电压稳定在 2.4 V,产氢速率保持在 1.84 mmol/h,产氢的法拉第效率接近 100%。这项工作同时考虑了水解离和 H* 吸附能在碱性 HER 中的重要性,为设计高活性碱性 HER 催化剂提供了一种策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


