Mayo Genetic Risk Models for Newly Diagnosed Acute Myeloid Leukemia Treated With Venetoclax + Hypomethylating Agent
IF 10.1
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
Patients with newly diagnosed acute myeloid leukemia (ND-AML) derive variable survival benefit from venetoclax + hypomethylating agent (Ven-HMA) therapy. The primary objective in the current study was to develop genetic risk models that are predictive of survival and are applicable at the time of diagnosis and after establishing treatment response. Among 400 ND-AML patients treated with Ven-HMA at the Mayo Clinic, 247 (62%) achieved complete remission with (CR) or without (CRi) count recovery. Multivariable analysis–derived hazard ratios (HR), including 1.8 for European LeukemiaNet (ELN) adverse karyotype, 4.7 for KMT2Ar, 1.7 for TP53MUT, 2.6 for KRAS MUT, and 2.1 for IDH2WT were applied to develop an HR-weighted risk model: low, intermediate, and high; respective median survival censored for allogeneic stem cell transplant (ASCT) (3-year survival) were “not reached” (67%), 19.1 (33%), and 7.1 months (0%). In patients achieving CR/CRi, adverse karyotype, KMT2Ar, KRASMUT, IDH2WT predicted inferior survival, allowing for a complementary response-stratified risk model. The model was externally validated and was shown to be superior to the ELN 2024 risk model (AIC 179 vs. 195 and AUC 0.77 vs. 0.69). Survival was inferior with failure to achieve CR/CRi or not receiving ASCT; 3-year survival for high-risk with or without ASCT was 42% versus 0% (p < 0.01); intermediate 72% versus 43% (p = 0.06); and low-risk 88% versus 78% (p = 0.53). The Mayo genetic risk models offer pre-treatment and response-based prognostic tools for ND-AML treated with Ven-HMA. The current study underscores the prognostically indispensable role of achieving CR/CRi and ASCT.
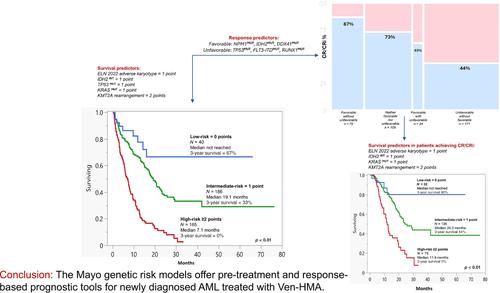
新确诊的急性髓性白血病(ND-AML)患者可从 Venetoclax + 低甲基化药物(Ven-HMA)治疗中获得不同的生存获益。本研究的主要目的是开发可预测生存期的遗传风险模型,该模型适用于诊断时和确定治疗反应后。在梅奥诊所接受Ven-HMA治疗的400名ND-AML患者中,247人(62%)获得了完全缓解,并伴有(CR)或不伴有(CRi)计数恢复。多变量分析得出的危险比(HR),包括欧洲白血病网络(ELN)不良核型的 1.8、KMT2Ar 的 4.7、TP53MUT 的 1.7、KRAS MUT 的 2.6 和 IDH2WT 的 2.1。在异基因干细胞移植(ASCT)(3年生存期)中,各自的中位生存期分别为 "未达到"(67%)、19.1个月(33%)和7.1个月(0%)。在达到CR/CRi的患者中,不良核型、KMT2Ar、KRASMUT、IDH2WT可预测较差的存活率,从而建立一个互补的反应分层风险模型。经外部验证,该模型优于 ELN 2024 风险模型(AIC 179 对 195,AUC 0.77 对 0.69)。未能达到CR/CRi或未接受ASCT的患者生存率较低;接受或未接受ASCT的高危患者3年生存率分别为42%对0%(p <0.01);中危患者72%对43%(p = 0.06);低危患者88%对78%(p = 0.53)。梅奥遗传风险模型为接受 Ven-HMA 治疗的 ND-AML 提供了治疗前和基于反应的预后工具。本研究强调了达到 CR/CRi 和 ASCT 在预后方面不可或缺的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
15.70
自引率
3.90%
发文量
363
审稿时长
3-6 weeks
期刊介绍:
The American Journal of Hematology offers extensive coverage of experimental and clinical aspects of blood diseases in humans and animal models. The journal publishes original contributions in both non-malignant and malignant hematological diseases, encompassing clinical and basic studies in areas such as hemostasis, thrombosis, immunology, blood banking, and stem cell biology. Clinical translational reports highlighting innovative therapeutic approaches for the diagnosis and treatment of hematological diseases are actively encouraged.The American Journal of Hematology features regular original laboratory and clinical research articles, brief research reports, critical reviews, images in hematology, as well as letters and correspondence.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


