Formation and Fate of Reactive Nitrogen during Biological Nitrogen Removal from Water: Important Yet Often Ignored Chemical Aspects of the Nitrogen Cycle
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
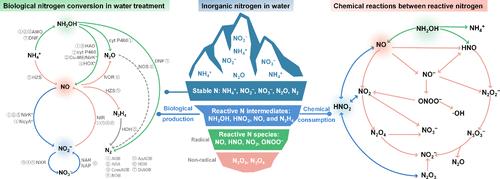
Hydroxylamine, nitrous acid, and nitric oxide are obligate intermediates or side metabolites in different nitrogen-converting microorganisms. These compounds are unstable and susceptible to the formation of highly reactive nitrogen species, including nitrogen dioxide, dinitrogen trioxide, nitroxyl, and peroxynitrite. Due to the high reactivity and cytotoxicity, the buildup of reactive nitrogen can affect the interplay of microorganisms/microbial processes, stimulate the reactions with organic compounds like organic micropollutants (OMP) and act as the precursors of nitrous oxide (N2O). However, there is little understanding of the occurrence and significance of reactive nitrogen during biological nitrogen conversions in engineered water systems. In this review, we evaluate the formation and fate of reactive nitrogen produced by microorganisms involved in biological nitrogen removal (BNR) processes, i.e., nitritation/nitrification, denitratation/denitrification, anammox, and the combined processes. While the formation of reactive nitrogen intermediates is entirely controlled by microbial activities, the consumption can be either biological or purely chemical. Changes in environmental conditions, such as redox transition, pH, and substrate availability, can imbalance the production and consumption of these reactive intermediates, thus leading to the transient accumulation of species. Based on previous experimental evidence, environmental relevance of reactive nitrogen in BNR systems, particularly related to abiotic N2O production and OMP transformation, is demonstrated.
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


