FcRn-dependent IgG accumulation in adipose tissue unmasks obesity pathophysiology
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Immunoglobulin G (IgG) is traditionally recognized as a plasma protein that neutralizes antigens for immune defense. However, our research demonstrates that IgG predominantly accumulates in adipose tissue during obesity development, triggering insulin resistance and macrophage infiltration. This accumulation is governed by neonatal Fc receptor (FcRn)-dependent recycling, orchestrated in adipose progenitor cells and macrophages during the early and late stages of diet-induced obesity (DIO), respectively. Targeting FcRn abolished IgG accumulation and rectified insulin resistance and metabolic degeneration in DIO. By integrating artificial intelligence (AI) modeling with in vivo and in vitro experimental models, we unexpectedly uncovered an interaction between IgG’s Fc-CH3 domain and the insulin receptor's ectodomain. This interaction hinders insulin binding, consequently obstructing insulin signaling and adipocyte functions. These findings unveil adipose IgG accumulation as a driving force in obesity pathophysiology, providing a novel therapeutic strategy to tackle metabolic dysfunctions.
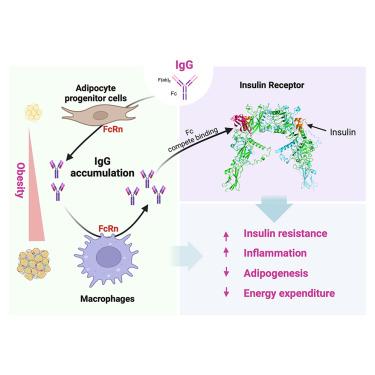
脂肪组织中依赖fcrn的IgG积累揭示了肥胖的病理生理
免疫球蛋白G (IgG)传统上被认为是一种血浆蛋白,可以中和抗原进行免疫防御。然而,我们的研究表明,在肥胖的发展过程中,IgG主要积聚在脂肪组织中,引发胰岛素抵抗和巨噬细胞浸润。这种积累是由新生儿Fc受体(FcRn)依赖的再循环控制的,分别在饮食诱导肥胖(DIO)的早期和晚期在脂肪祖细胞和巨噬细胞中进行。靶向FcRn可消除IgG积累,纠正DIO的胰岛素抵抗和代谢变性。通过将人工智能(AI)建模与体内和体外实验模型相结合,我们意外地发现了IgG的Fc-CH3结构域与胰岛素受体外结构域之间的相互作用。这种相互作用阻碍胰岛素结合,从而阻碍胰岛素信号传导和脂肪细胞功能。这些发现揭示了脂肪IgG积累在肥胖病理生理中的驱动作用,为解决代谢功能障碍提供了一种新的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


